Chlorine vs. Bromine in Indoor Pools
While chlorine dominates the aquatics market as the most popular residual sanitizer, many pool operators have asked about chlorine alternatives. The main chlorine alternative is bromine, and this article will break down the pros and cons of each, so you can make an informed decision about what is best for your pool.
Covered in this article:
- Why do pools need a residual sanitizer?
- Chlorine
- Types of chlorine
- Chemistry of chlorination
- How chlorine kills
- Chloramine byproducts
- Compatibility with secondary systems
- Chlorine pros and cons
- Bromine
- Types of bromine
- Chemistry of bromination
- Bromine byproducts
- Bromamines
- Trihalomethanes (THMs)
- Bromates
- Compatibility with secondary systems
Why do pools need a residual sanitizer?
There seems to always be an interest in discovering a way to have a "chlorine-free pool." And while there are several chlorine alternatives, the real goal is to have a pool without a primary residual sanitizer. And we're here to tell you we disagree with that goal. While it sounds nice to have a chlorine-free pool, having a residual sanitizer–usually chlorine–is a must.
Related: Pool Water Chemistry Resources

A residual sanitizer means the sanitizer is dissolved in the water and circulates throughout the pool and its equipment. It can be tested regularly. A residual sanitizer is the first line of defense against recreational water illnesses (RWIs), germs and bacteria. A secondary sanitizer would be a system like UV, Ozone or AOP. They are secondary because they do not create residual that stays in the pool. These systems are point-of-contact.
Chlorine is the most popular residual sanitizer used in pools. But the second-most popular is bromine. Both are halogen elements that disinfect water, and they behave in a similar way.
Chlorine
Of all the swimming pool disinfection methods, chlorine is the most popular. At room temperature, elemental chlorine exists as Cl2, which is a greenish yellow gas that is highly toxic. Chlorine gas is rarely used in pools anymore, but it was once widely used. Today, there are several chlorine products, including saltwater chlorine generators, which actually create chlorine gas in the water using electrolysis.1
Types of chlorine
The most popular types of chlorine in commercial pools–in order of popularity–are:
- Liquid Chlorine (sodium hypochlorite)
- Cal Hypo (calcium hypochlorite)
- Trichlor (Trichloro-S-Triazinetrione)
- Salt chlorine generator
Other types of chlorine exist, but they are rarely used in commercial pools. These include dichlor, lithium hypochlorite, and chlorine gas (which is outlawed in most states nowadays). For more detailed information on each of these chlorine types, read this Orenda article. All types of chlorine will dissolve in water and yield the same active forms of free chlorine. The only differences are the byproducts of these reactions and each chlorine type's temporary pH impact.
The chemistry of chlorination
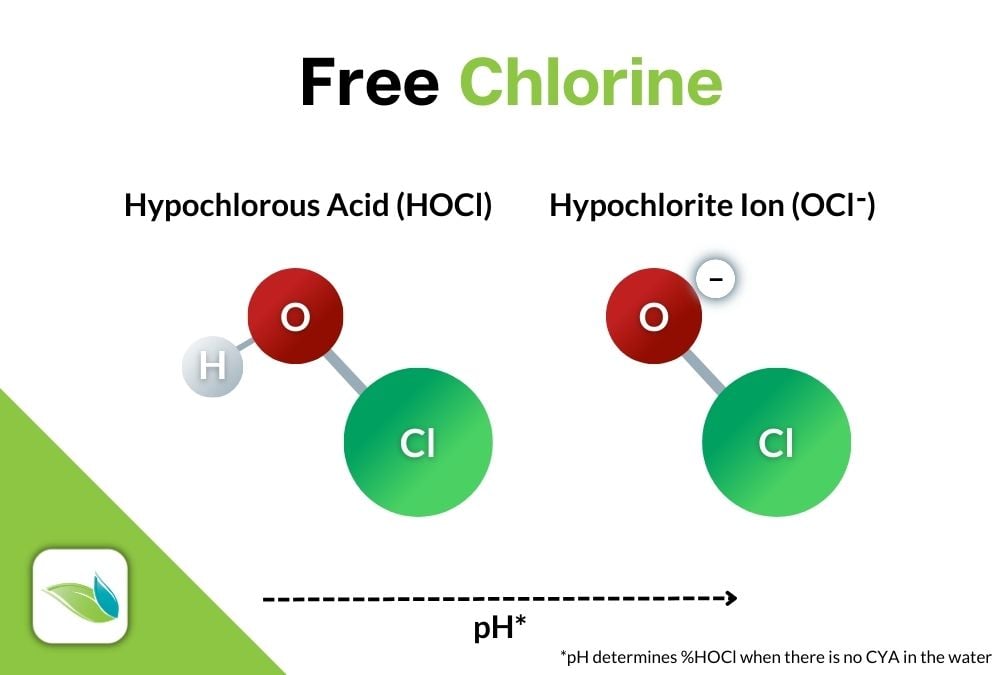
When added to water, every type of chlorine will convert into two forms of Free Chlorine (FC):
- The strong, killing form of chlorine, Hypochlorous Acid (HOCl).
- The weaker, slower form of chlorine, Hypochlorite Ion (OCl-).
Here's the chemistry of this quick reactions that happen in succession:
Cl2 + H2O → HOCl + HCl
Chlorine(gas) + Water → Hypochlorous acid + Hydrochloric acid
Then, almost immediately, a schism happens within HOCl itself, where Hydrogen dissociates from HOCl to create OCl-:
HOCl ⇌ H+ + OCl-
Hypochlorous acid ⇌ Hydrogen ion + Hypochlorite ion
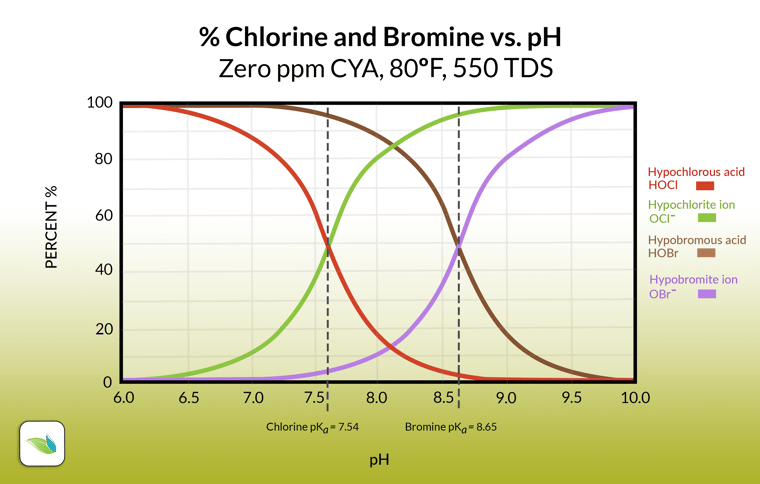
This dissociation of Hydrogen from HOCl stays in equilibrium, as a function of pH. In other words, pH controls this equilibrium, and thus the strength of chlorine (%HOCl)–but only in pools without cyanuric acid (CYA)!
The red line represents HOCl. Without CYA in the water, pH determines the %HOCl, and therefore the "strength" or "speed" of chlorine. The lower the pH, the higher the %HOCl, and therefore the better chlorine can perform. But if there is CYA in the water, the chemistry is fundamentally different.2
How chlorine kills
Chlorine in its active form (HOCl) has a neutral charge, which attracts negatively-charged contaminants. Chlorine penetrates the cell wall of the organism and destroys it from within, preventing it from reproducing.3 This is called sanitization (99.9% reduction of all living microorganisms). Disinfection is a bit more specific, in that its just killing harmful microorganisms, so it would not include things like algae.
Chloramine byproducts
When chlorine combines with nitrogen compounds, disinfection byproducts (DBPs) are created.4 These byproducts are called combined chlorine (CC), and can be measured by subtracting free available chlorine (FAC) from total available chlorine (TC).
TAC - FAC = CC
Inorganic nitrogen is usually in the form of ammonia. When it is oxidized by chlorine, it creates chloramines:
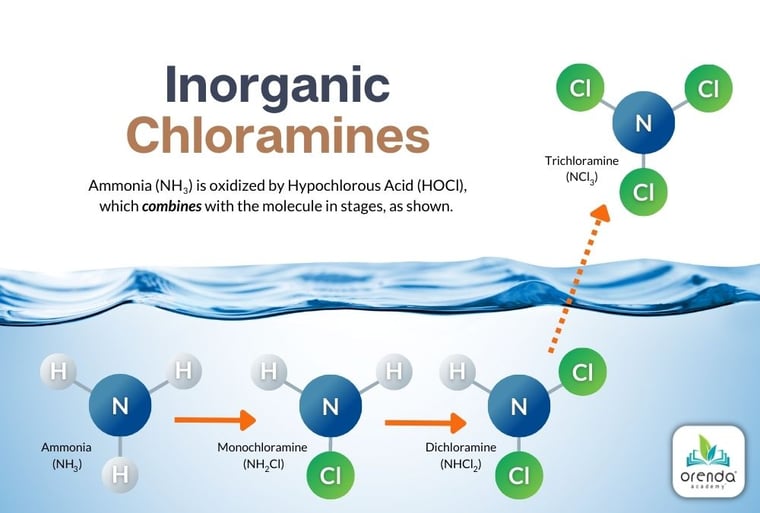
If the nitrogen compound also contains carbon, like urea, the chemistry is more complex. The byproducts are not just mono-, di-, and trichloramines...there can be hundreds of different variations of chloro-organic compounds. To simplify all of these, we in the industry use the term chloramines generically to cover all of these byproducts.
Related: Ways to optimize chloramine removal
Related: Ways to reduce combined chlorine
Chlorine compatibility with secondary systems
As far as we know, all types of chlorine used in commercial pools are compatible with secondary disinfection systems. One caveat is that UV systems can degrade some chlorine as water passes through the system. In indoor pools without cyanuric acid, free chlorine is unprotected from UV degradation. Generally speaking, this chlorine reduction is not very noticeable because water is moving quickly through the UV system.
Chlorine pros and cons
Chlorine has many advantages over bromine. First, it is more powerful and effective at killing and oxidizing than bromine. It is also far more affordable and widely available. And for outdoor pools–which our services do not apply to–chlorine has a distinct advantage in that it can be stabilized against sunlight degradation. Therefore, chlorine is the sanitizer of choice for outdoor pools.
On the downside, chloramine byproducts (including chloro-organic DBPs) are harmful, irritating to swimmers, and corrosive. Each type of chlorine leaves behind inert byproducts, but those byproducts accumulate over time...namely salt.
Bromine
After chlorine, bromine is the most widely-used alternative sanitizer in pools and spas. It is more commonly used in spas and hot tubs than pools. And since it cannot be stabilized against sunlight degradation, it should not be used in outdoor pools. At room temperature, elemental bromine is a smelly, brown liquid.
Both bromine and chlorine are part of the halogen group on the Periodic Table of Elements. Bromine is below chlorine, making it less reactive. Because of this, chlorine can actually oxidize bromide ions into bromine. This is important to understand, and we'll elaborate in a moment.
Types of bromine
Bromine is sold as tablets, briquettes, granules, and in liquid form. Each of these products is different and has a different pH impact in the water.
The most common bromine products are:
- BCDMH (bromo-chloro-5,5-dimethylhydantoin) tablets
- DBDMH (dibromo-5,5-dimethylhydantoin)
- BCDMH + DCDMH + DCEMH
- Liquid bromine
BCDMH tablets are the most popular bromine product on the market. They have a moderately acidic pH of about 4.5-4.8. BCDMH+DCDMH+DCEMH has a lower pH around 3.6, and DBDMH (dibromo) has a relatively neutral pH of about 6.6.
The chemistry of bromination
Just like with chlorine, when any of these bromine types dissolve in water, they all produce Hypobromous acid (HOBr), which is the active form of bromine. As the similarity in names indicates, this is bromine's equivalent to chlorine's Hypochlorous acid (HOCl). So when bromine dissolves in water it looks like this:
Br2 + H2O → HOBr + HBr
bromine + water → Hypobromous acid + Hydrobromic acid
HOBr is bromine's equivalent to chlorine's HOCl. And like HOCl, HOBr will dissociate into its much weaker counterpart, Hypobromite ion (OBr-), based on pH:
HOBr ⥄ H+ + OBr-
Hypobromous acid dissociates into Hydrogen ion and Hypobromite ion
The reason for the (⥄) instead of equal dissociation is that this equilibrium happens at a higher pH than pools and spas normally operate under. So most of the active bromine in water will be HOBr relative to OBr-. See the difference between chlorine and bromine in the graph below:
And just like HOCl gets reduced down to chloride ions (Cl-), HOBr gets reduced down to bromide ions (Br-) when it oxidizes or kills contaminants in water.
But here's where the similarities to chlorine end. While chloride ions are inert and useless, bromide ions can be oxidized back into bromine itself. That bromine once again becomes HOBr and can continue oxidizing and disinfecting water. As mentioned a moment ago, this can happen because bromine is beneath chlorine on the periodic table.5 And it's not just chlorine that can oxidize bromide ions back to bromine. Secondary oxidizers like ozone and AOP can also do it, as well as a non-chlorine oxidizing shock like potassium monopersulfate.6
Just like chlorine (and other halogens), bromine attacks cell walls and destroys pathogens from within. What happens afterward is different than chlorine because of bromine regeneration. This can get complex, so we'll simplify it. First, a visual of the bromine cycle.
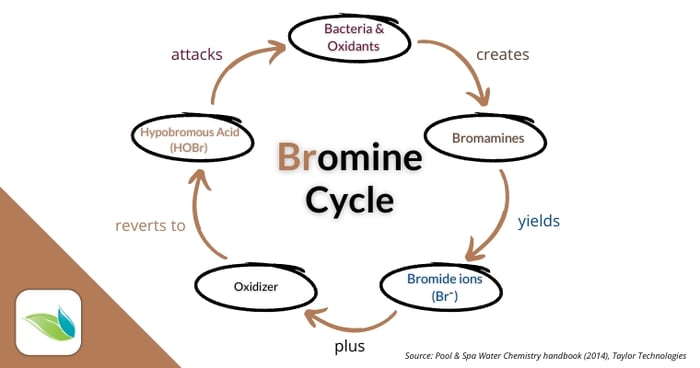
Hypobromous acid attacks germs and oxidants. For the most part it destroys these things, though just like chlorine, bromine is inefficient at oxidizing non-living organics and oils. And when it oxidizes nitrogen compounds, bromamine byproducts are created. This is very similar chemistry to chlorine creating chloramine byproducts.
Bromamines (like chloramines) have some disinfection power, and eventually get reduced down to bromide ions. The bromine that attacked germs, metals and other oxidants without nitrogen also get reduced down to bromide ions (Br-). These bromide ions can then be oxidized by a chlorine shock, non-chlorine shock, or secondary oxidation system to create Hypobromous acid once again.7 Then the cycle repeats itself.
Bromine byproducts
Like chlorine, bromine leaves behind byproducts in the water. Some of them are harmless, while others are not.
Bromamines
Unlike chlorine, combined bromine cannot be measured. Just measure total bromine. And that's okay because bromamines are decent sanitizers, so there's really no reason to distinguish them in a test.
Some sources say bromamine byproducts do not cause irritation or smell bad. And to us, that's a matter of opinion, because we know many swimmers who struggle to breathe in indoor bromine pools. It is noticeably less harsh than chlorine, but to some swimmers it can seem worse. It depends on the swimmer.
Trihalomethanes (THMs)
The bigger issue in bromine pools is actually Trihalomethanes (THMs). In chlorine pools, only one of the four trihalomethanes (chloroform) can be created, because bromine is not present in the water. But if you are using a product like BCDMH, which contains both bromine and chlorine, all four types of THMs are possible in your water:
.jpg?width=760&height=700&name=trihalomethanes%20(THM).jpg)
The bromamine and THM byproducts that off-gas from bromine pools behave similarly to chloramine vapor, in that they are heavier than oxygen. Therefore the HVAC system for an indoor pool should be designed the same–with source-capture exhaust–regardless of whether the pool uses chlorine or bromine.
Related: Indoor Pool HVAC design resources
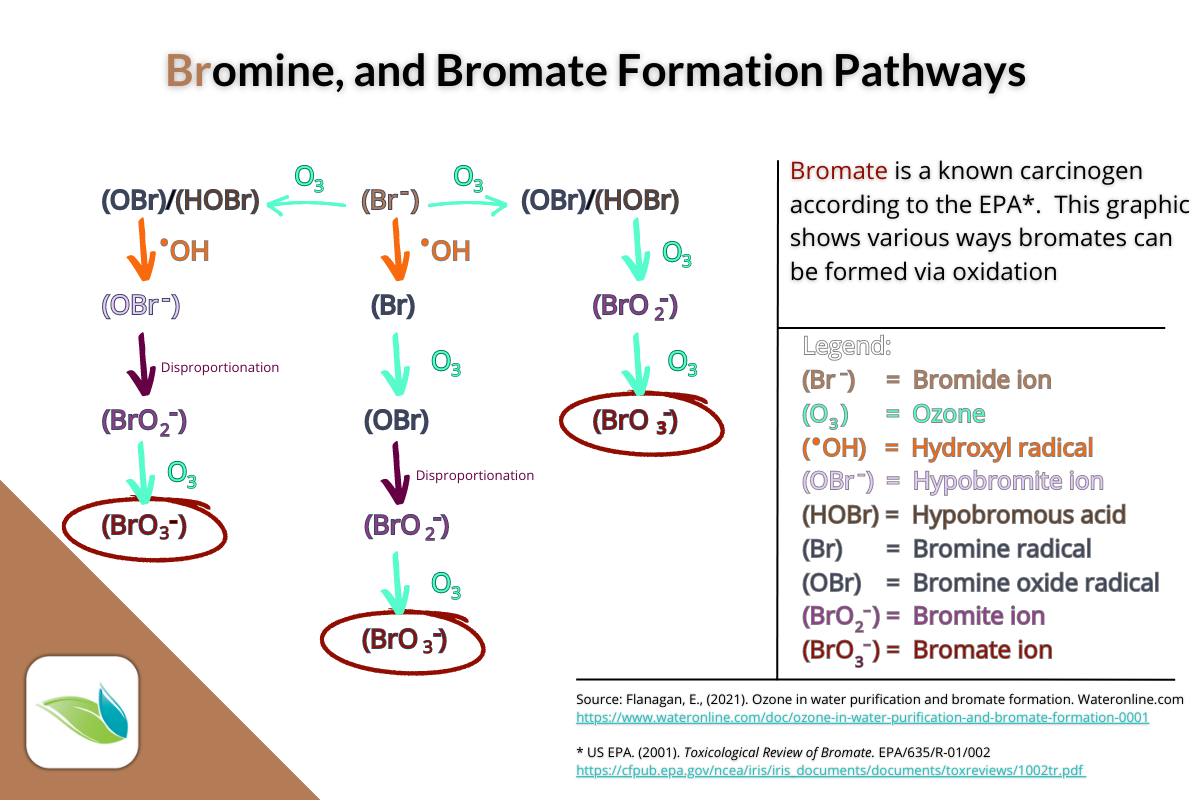
Bromates
Another byproduct of bromine is created if bromine is exposed to direct sunlight, or when bromide ions are oxidized by ozone. It's called Bromate (BrO3-), and it is classified as a known carcinogen in drinking water by the EPA.
Bromates can be formed in numerous ways in a bromine pool or spa. Interaction with ozone, hydroxyl radicals, hydrogen peroxide or UV light can all create harmful bromate ions.
Bromine compatibility with secondary systems
Prior to writing this article, we would have encouraged the use of Ozone and AOP systems on bromine pools because the oxidation recharges bromine. And that's true, except we just learned (while researching this) about ozone and hydroxyl radicals creating harmful bromates too.
UV light also creates bromates, due to photolysis. So UV systems are not to be used on bromine pools either.8 And that means no bromine in outdoor pools either due to direct sunlight, and the fact that bromine does not have a stabilizer (think a cyanuric acid equivalent, but for bromine).
That leaves only a few options for secondary systems, and none of them are registered as secondary disinfection systems. The first type of secondary system, which works extraordinarily well in pools, is Hyper-dissolved Oxygen (HDO). We have talked about HDO before. The second is Hydro-dynamic Cavitation (HDC). We have not conducted studies on how well these systems perform in bromine pools, but we know that they should be safe and compatible with bromine, unlike the other secondary systems.
Conclusion
Both chlorine and bromine are halogen elements that can disinfect water and oxidize non-living contaminants. Chlorine is comparatively stronger, as it is one row above bromine on the Periodic Table of Elements. This also means chlorine (and other oxidizers) can oxidize bromide ions back into hypobromous acid, which "recharges" bromine. This is one nice feature of bromine. But it's a two-edged sword.
If bromide ions (Br-) get oxidized enough, they can also form harmful bromates. Bromates can also be created by UV light. Since bromine does not have a stabilizer against UV degradation (unlike chlorine, which has cyanuric acid), bromine should not be used in outdoor pools or indoor pools with UV systems. In fact, the only secondary systems fully compatible with bromine are not technically secondary disinfection systems at all, but they are good supplemental systems: Hyper-dissolved oxygen (HDO), and hydro-dynamic cavitation (HDC).
In summary, bromine is a decent option if you have a warm-water indoor pool with a low-to-moderate bather load. It performs well in spas, and its bromamine byproducts are less irritating and corrosive than chloramines. That being said, bromine pools will have more trihalomethanes (THMs), so there's a trade-off. If you are going to have an indoor bromine pool, we recommend shocking with potassium monopersulfate, which is a non-chlorine oxidizer. This can recharge bromine without creating bromates.
In terms of air quality, both chloramine and bromamine byproducts off-gas and should be dealt with appropriately with source-capture ventilation, as they are heavier than oxygen and stay low in the room.
1 Contrary to popular belief, saltwater pools ARE chlorine pools. Salt systems create chlorine using electricity, which converts chloride ions (Cl-) in saltwater into chlorine gas (Cl2⇡). Other byproducts include sodium hydroxide (NaOH), Hydrogen gas (H2⇡), and the turbulence created by the hydrogen gas bubbles releases carbon dioxide from solution (CO2⇡), causing the pH in the pool to rise.
2 Cyanuric acid (CYA) stabilizes chlorine and protects it from sunlight degradation. But because the focus of this website is indoor commercial pools, there should not be any CYA in the water to begin with. We're just including this to make the point that pH does not always control the strength of chlorine. Just in pools without any CYA. That being said, you should at least be aware that CYA changes the chemistry of chlorination substantially, including the production of chloramines. Read this to learn more.
3 Lowry, Robert. (2016). IPSSA Basic Training Manual, 2016 revised edition. Ch.5 (pg. 57).
4 The name "disinfection byproducts" is what the industry uses, but technically it's not an accurate name for what we're talking about. DBPs are actually oxidation byproducts of chlorine attacking nitrogen compounds. And if the nitrogen compound contains carbon bonds, it's an organic nitrogen compound (like urea) that creates even more complex DBPs called chloro-organic compounds.
5 In the halogen group of elements on the Periodic Table, higher elements in the group can oxidize lower elements. Chlorine can oxidize bromide ions into bromine, just like both bromine and chlorine can oxidize iodide ions into iodine. Theoretically fluorine could oxidize chloride ions into chlorine, but according to this source, fluorine is too powerful of an oxidizer that it actually oxidizes water itself, so aqueous reactions cannot take place. We just learned this while researching for this article. And it makes sense now. This must be why fluorine is not used in swimming pools, and the next-best halogen (chlorine) is the most dominant residual sanitizer on the market.
6 Lampre, I., Marignier, J.L., Mirdamadi-Esfahani, M., et.al. (2013). Oxidation of Bromide Ions by Hydroxyl Radicals: Spectral Characterization of the Intermediate BrOH•-. Journal of Physical Chemistry. 117, 5. (pp. 877-887). https://doi.org/10.1021/jp310759u
7 We should clarify that ozone can both "recharge" bromine by oxidizing bromide ions, and it can also convert bromide ions into harmful bromates. This is complex chemistry, but in summary, ozone can oxidize bromide ion (Br-) into hypobromous acid (HOBr) and hydrobromic acid (OBr-). But if more ozone continues to oxidize HOBr, bromite ion (BrO2-) will be created. If still MORE ozone oxidizes bromite, it will become bromate (BrO3-). A good explanation and graphic of this process can be found here.
8 Fang, J., Zhao, Q., Fan, C., et.al. (2017). Bromate formation from the oxidation of bromide in the UV/chlorine process with low pressure and medium pressure UV lamps. Chemosphere, vol. 183 (pp. 582-588). https://doi.org/10.1016/j.chemosphere.2017.05.136

 By
By