Ozone for Swimming Pools
Continuing our conversation on secondary disinfection and oxidation systems, today we are discussing ozone (O3). Ozone is a powerful oxidizer and disinfectant for water, and is used in a wide array of industries from drinking water to food processing.
First and foremost, we are not ozone experts, and we do not pretend to be. This article is aggregating information available online (which we will hyperlink to) and from speaking directly with the aquatics industry's leading ozone expert, Beth Hamil. Thanks to Beth, we also have free, downloadable PDFs of scientific documents and white papers about ozone. The point is, we are citing our sources, and aim to give you the most factual and verified information available, while simplifying it so it is easier to understand. Some of this technology is complicated.
Related: Pool Water Chemistry Resources
How is Ozone Generated?
There are three ways that ozone is created–at least in the world of aquatics. They are:
-
- Ultraviolet Light (UV)
- Corona Discharge (random discharge)
- Microplasma (uniform discharge)
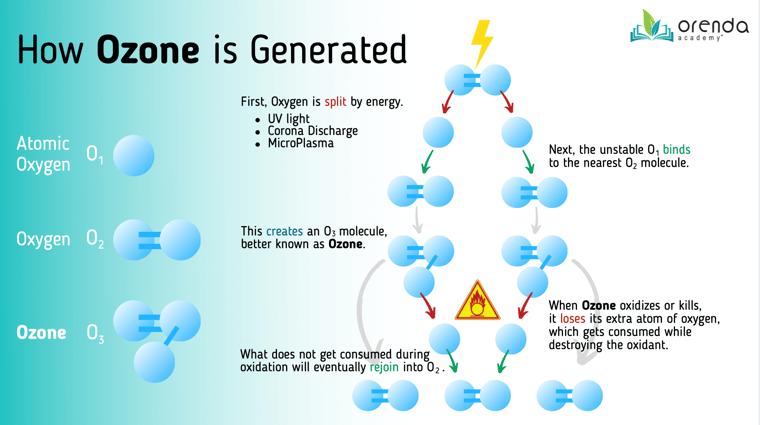
All three systems are designed to concentrate enough energy to split Oxygen (O2) into two unstable O1 atoms. These O1 atoms will bind to other O2 molecules and form O3, which is Ozone. Here's a visual diagram:
Ultraviolet-Generated Ozone
UV light–at a specific wavelength (~185 nm)–breaks the bond that holds Oxygen (O2) together. Ambient air flows through the UV chamber, and when it comes out the other side, some of the oxygen has been broken apart and has formed ozone (O3), which is still in gas form. The ozone gas is then injected into the water, where it destroys oxidants and kills pathogens.
Corona Discharge Ozone
Corona Discharge (CD) works using electricity to break apart O2 molecules in the air that passes through the chamber (in gas form). From Ozonize:
At the heart of a corona discharge ozone system is the dielectric. The electrical charge is diffused over this dielectric surface, creating an electrical field, or “corona”.
CD produces a substantially higher quantity and concentration of ozone than UV does. Its energy costs are also comparatively lower. The CD process is considered "random discharge", and can be set up in varying strengths and frequencies, depending on the application and the amount of ozone needed.
CD ozone systems also produce heat, due to the electrical activity, so ventilation and some cooling mechanism is required.
Microplasma Ozone
Microplasma ozone is the most recent development in ozone generator technology. Just like Corona Discharge, it uses electricity to split O2 molecules. It just does so more effectively.
Rather than a "random discharge" like CD, Microplasma creates a "uniform glow discharge", which offers much higher efficiency. This means less energy needed, while creating significantly more ozone. Systems like the Microplasma Ozone MPO5 for swimming pools also come with an oxygen concentrator and an internal dehumidifier to optimize efficiency. Rather than splitting apart oxygen in ambient air–which is only about 21%–an oxygen concentrator gets the O2 percentage over 90%.
How Does Ozone Disinfect and Oxidize?
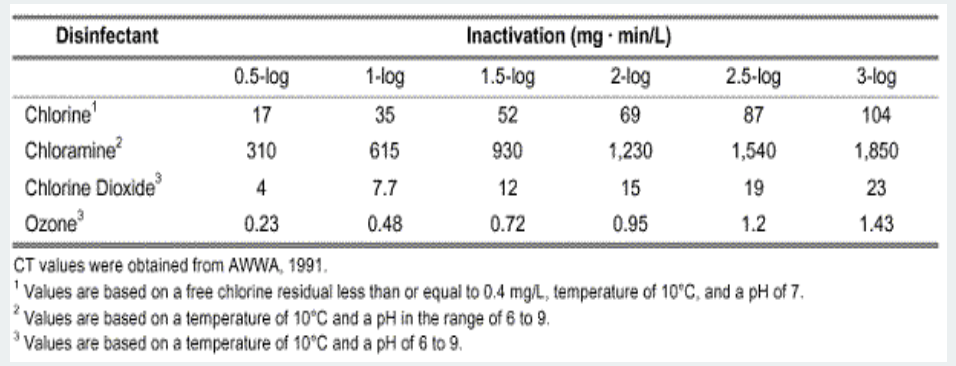
Ozone disinfects when it smashes into living microorganisms. The third Oxygen atom breaks off and ruptures the cell wall of the microorganism; destroying it almost instantly. It is a far stronger disinfectant than chlorine. To give you an idea of the order of magnitude difference, let's compare ozone vs. chlorine.
Disinfection strength is measured by Contact Time (CT). The higher the CT value, the slower and weaker the disinfectant. Here's a chart showing CT values for killing Giardia cysts, from this source:
Notice how small the CT values are for Ozone compared to Chlorine. Chlorine is nearly 100 times slower.
Even the highly chlorine-resistant parasite Cryptosporidium Parvum has been proven to be killed by Ozone effectively.1 One pool ozone system demonstrated a 4.1-log reduction of C. Parvum in a single pass.2 Ozone was shown to be incomparably stronger than chlorine against Crypto (15,300 to 1), and it also destroys biofilms that it comes in direct contact with.
And these studies and facts were not produced by the manufacturers. Ozone systems must be independently certified and validated by NSF International and other third-party testing organizations, which means it is about as proven as a technology can be.
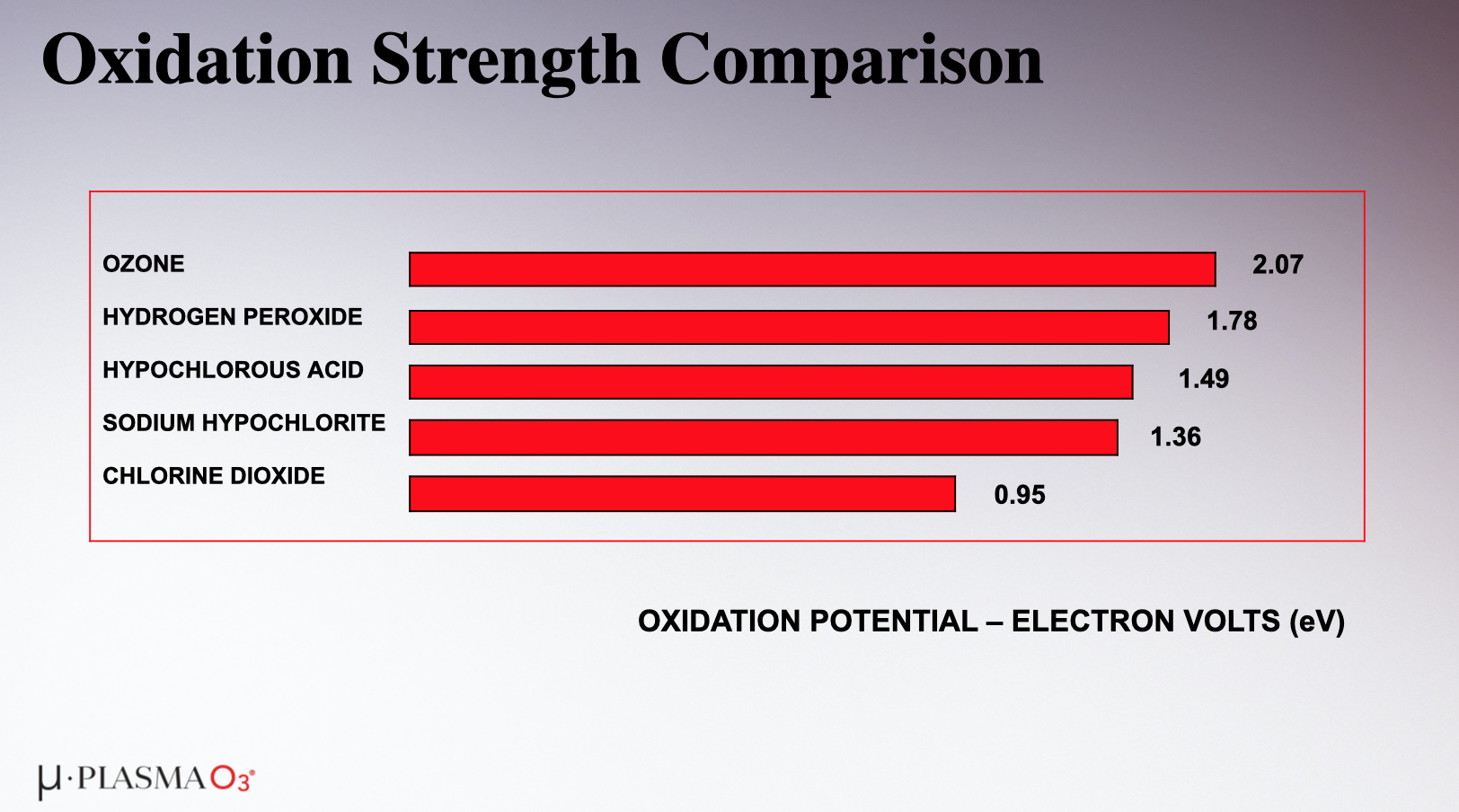
Ozone oxidizes incredibly effectively too. Much like disinfection, the third Oxygen atom smashes into the oxidizable substance, and that's that. Ozone is powerful enough that it can destroy monochloramine and dichloramine on contact, as well as urea and other nitrogen compounds. This is a big deal, because while UV systems can deactivate chloramines, they cannot oxidize the precursors to chloramines (like urea). Ozone can. Here is a chart comparing oxidation strengths:
There is another oxidizer that is not shown on this chart that is even higher than ozone: a Hydroxyl Radical. But that's all well and good, because when it is dissolved in water, ozone exists in equilibrium. That means approximately half of the O2 that is split will become ozone, and the other half will be hydroxyl radicals.
Ozone and Indoor Air Quality
Ahh yes, of course, indoor air quality. The issue we care about most. From our personal experience, secondary sanitizer systems like ozone help water quality immensely. Unfortunately, they do not seem to have enough of an impact to prevent indoor air quality problems on their own. Yes, ozone does help, but in our personal swimming experience, even the pools with ozone can have chloramine issues. Sure, ozone will easily destroy what it comes in contact with...but remember that ozone systems are point-of-contact.
Chloramines off-gas in the natatorium and become an air problem. Many mono- and dichloramines never have a chance to be destroyed by ozone, and instead turn into trichloramine and off-gas. Without a doubt, however, ozone has one key advantage over UV when it comes to indoor air quality. Ozone destroys precursors to chloramines, like urea, other nitrogen compounds, and non-living organics. UV can only break down formed chloramines...it does not oxidize.
So while ozone is a proven badass secondary disinfectant and oxidizer system, its impact on indoor air quality is limited to treating the water that circulates through the pump room. While some ozone can get into the pool, there are codes that require de-gassing chambers to avoid inhalation of ozone by swimmers.
We hope this article helps explain how ozone works, and how effective it is at oxidation and disinfection.
1 Rennecker, J. L., Mariñas, B. J., Owens, J. H., & Rice, E. W. (1999). Inactivation of Cryptosporidium parvum oocysts with ozone. Water Research, 33(11), 2481–2488. doi: 10.1016/s0043-1354(99)00116-5
2 Donofrio, Aridi, Saha, Bechanko, Schaefer, Bestervelt & Hamil (2013). Laboratory validation of an ozone device for recreational water treatment. Journal of Water & Health, 11 (2) 267-276. https://doi.org/10.2166/wh.2013.198

 By
By