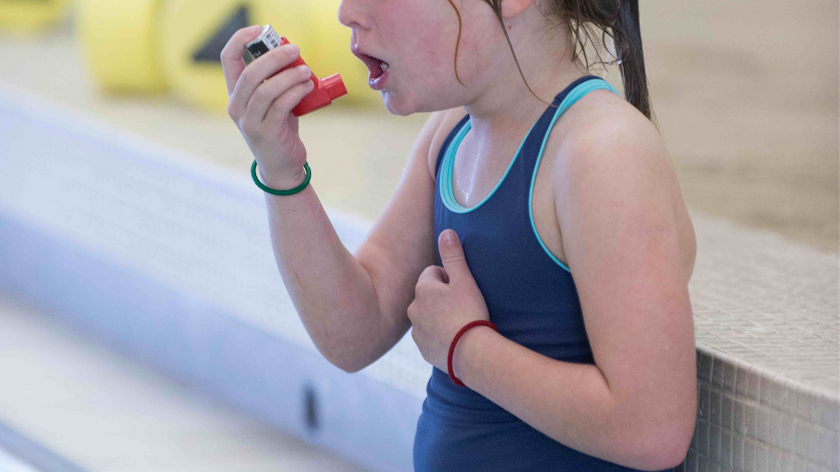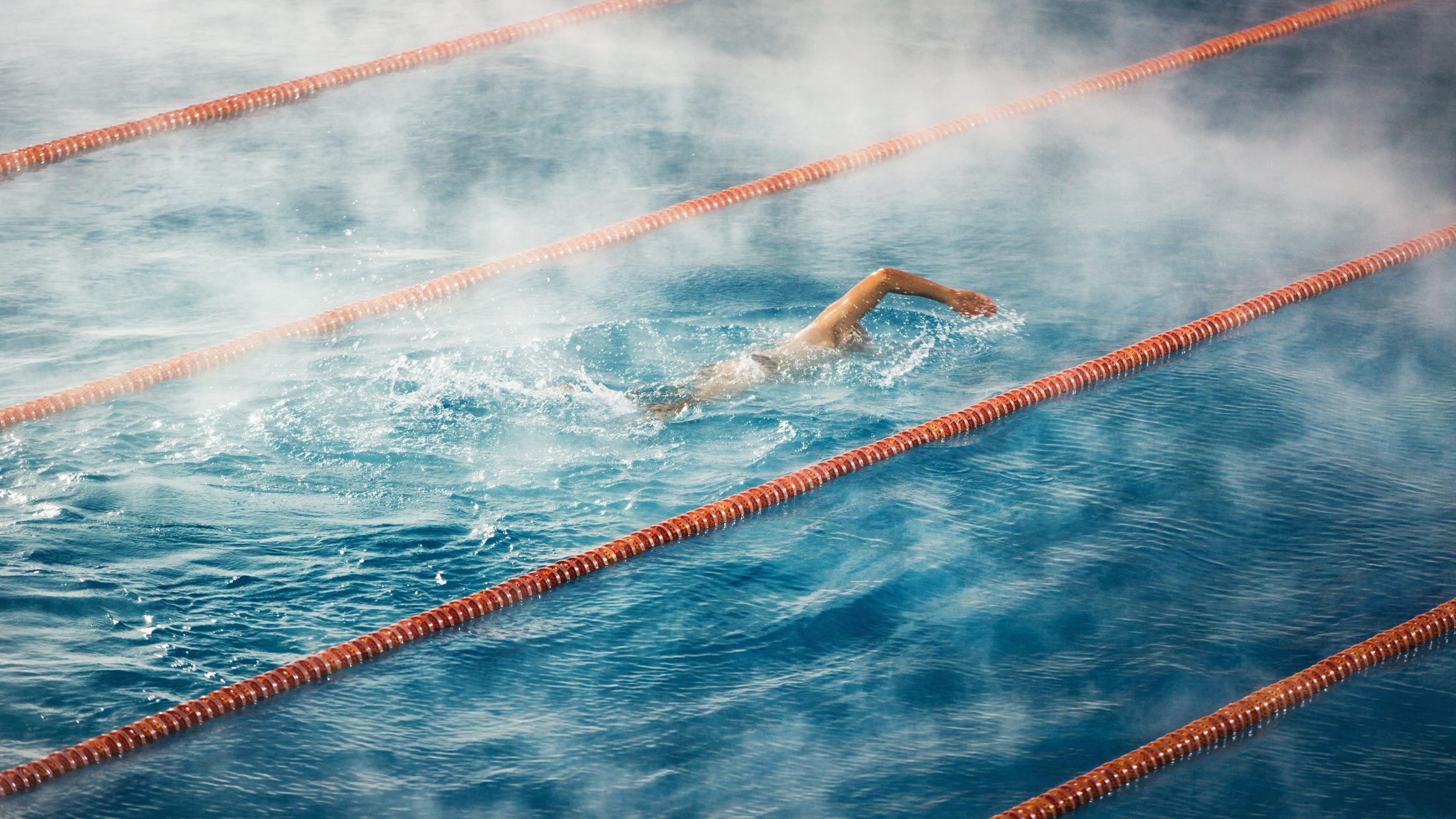Chloramine Vapor
Indoor air quality in natatoriums has many variables and moving pieces, but at the end of the day, it all boils down to one thing: how effectively can the air handling system remove vaporized pool air laden with chloramines?
Covered in this article:
- What is chloramine vapor?
- How are chloramines produced?
- Vapor, humidity, expansion
- How to address chloramines...
- ...in the water
- ...in the air
- Conclusion
What is Chloramine Vapor?
Chloramine vapor is the airborne form of trichloramine and other disinfection byproducts (DBPs), which off-gas from swimming pools or other treated water sources.
To clarify, the term chloramines is also used broadly to include other airborne disinfection byproducts beyond just inorganic chloramines (mono-, di-, and trichloramines). The broad term chloramines includes things like chloroform (also called methyl trichloride, or trichloromethane, CHCl3), the harmful trihalomethane, and cyanogen chloride.
Basically, any DBPs that off-gas from a swimming pool are commonly included when talking about chloramines, even though it's not technically accurate.1
Chloramine vapor occurs when water evaporates (vaporizes into the air), and that moisture in the air is laden with chloramines. This is important because chloramines change the chemistry of that moisture; it becomes acidic and corrosive to metals, as well as being harmful to people.

How are chloramines produced?
Inorganic chloramines are produced when ammonia gets oxidized by chlorine. Specifically, it's when the strong form of aqueous chlorine, hypochlorous acid (HOCl) interacts with ammonia (NH3).
This reaction unfolds in several steps. At a 5:1 weight ratio (HOCl to NH3), HOCl replaces a hydrogen with a chloride (Cl), creating monochloramine (NH2Cl). More chlorine (10:1 weight ratio) will eventually replace another Hydrogen, creating dichloramine (NHCl2). And still more chlorine (15:1 weight ratio)will eventually replace the final Hydrogen, creating nitrogen trichloride, more commonly known as trichloramine (NCl3).
These reactions are the crux of what is called combined chlorine. Chlorine combines with nitrogen compounds. And what we just outlined was only inorganic chloramines...these reactions are straightforward. It gets far more complex when there are organic nitrogen compounds in water, like urea.

The point is this: it takes a lot of chlorine to handle these nitrogen compounds. The more complex the molecule, the more chlorine it takes to destroy, and the more variations of byproducts can be produced. And hundreds of these byproducts can go airborne when they off-gas.
So when we say chloramine vapor, we're talking about many substances in the air that we do not want anyone to breathe.
Vapor, Humidity and Expansion
Let's quickly cover some glossary terms for this subject.
Water vapor is evaporated water in the air. Humidity is a measurement of the amount of water vapor in the air. Absolute humidity is the amount currently in the air, and relative humidity (RH) is the amount of water vapor compared to how much vapor the air could potentially hold.
Both heat and humidity rise and expand, so it is common for natatoriums to have ceiling exhausts. While they make sense from a dehumidification standpoint, ceiling exhausts are a common mistake in natatorium design. Sure, they exhaust the hottest, most humid air in the room, which can reduce the moisture load on the dehumidifier. But the problem with natatorium air quality is not water vapor, it's chloramine vapor.
Unlike water vapor, chloramine vapor does not rise. Chloramines and other DBPs are heavier than oxygen. They stay low in the natatorium, just above the surface of the pool in what we call the breathing zone. Another term for this is the chloramine bubble, coined by Randy Baxter in this article. The more chloramines and DBPs off-gassed, the denser the bubble, and the more displacement of fresh air for swimmers to breathe. It's not a good thing.
Left alone, chloramine vapor will eventually fill the natatorium from the bottom up. With poor HVAC design and a high return intake, this can lead to a stratification problem, where chloramines just build and build. Notice that chloramines do not rise and expand like humidity does.

A low return intake can compound a chloramine problem too, by recirculating them. In both scenarios, the issue is that neither return location captures or removes chloramines effectively. Normally the exhaust blower is within the return air path, and it's just a fan; a fan without the ability to separate good air from chloramine vapor. Stratification and recirculation are different, but they are both problems. Which leads us to some solutions...
How to address chloramines
Eventually, the concentration of trichloramine in the water will want to match the concentration in the air, per Henry's Law. But natatoriums are large buildings with plenty of space for chloramine vapor to spread. Henry's Law is not much of a concern in the real world, because trichloramine concentrations would need to be significant to overpower the vapor pressure pushing outward from the pool. But we're getting into the weeds now.
It takes a multi-pronged approach to handle chloramine vapor.
Chloramines in the water
From the water quality side, it is important to address the oxidant demand head-on. If chlorine is by itself in its battle against bather waste (including nitrogen compounds like urea), more byproducts are bound to happen. This can be a two-pronged approach.
First, encourage swimmers to take bathroom breaks and to shower before entering the pool. this can help in a major way, but it's hard to enforce. Old habits die hard, but a cultural change is possible if everyone commits to it.
Second, maximize the removal of DBPs (and more importantly, their precursors!) while they are still in the water. This can be accomplished by supplementing chlorine against the oxidant demand. Secondary systems like ozone and AOP destroy just about anything that passes through their area of effect. Medium pressure UV destroys formed chloramines and inactivates living pathogens, but it is not an oxidizer. Finally, supplement chlorine with enzymes, which digest non-living organics, oils and other hydrocarbons. These things make up the vast majority of chlorine demand, so why not remove them from water as efficiently as possible?
Chloramines in the air
Once chloramines go airborne, they are now an air problem and must be handled accordingly. We strongly recommend designing natatoriums with air quality as the foremost priority. Design with end users (swimmers, lifeguards, coaches, etc.) in mind.
It is essential to have an air system that is designed to match the swimming pool and the room it's in. A good airflow design and properly-located return intakes are essential.
Related: 21st Century Natatorium Design Guide, by Desert Aire
Equally important is using source-capture exhaust to capture and remove chloramine vapor from the room. Source-capture exhaust allows the system to exhaust the highest concentration of chloramine vapor, while the rest of the room can be recirculated and conditioned by the pool dehumidifier. Capturing heavy chloramines and trihalomethanes at their source keeps them from spreading around, and helps a pool maintain excellent air quality 24/7.
Conclusion
Chloramine vapor is an inevitable contaminant in indoor swimming pools. Outdoor pools have it too, but because it is outdoor, the abundance of fresh air means we hardly notice. We can try to mitigate chloramine vapor production via water chemistry strategies, but there will always be some that makes it out into the air. And while water vapor rises and expands, chloramine vapor does not, because it is heavier than oxygen. This makes chloramines predictable and easy to capture and remove with a targeted source-capture exhaust system.
1 As an aside, in bromine pools and spas, the predominant DBPs are called bromamines, which off-gas into bromamine vapor. But since the overwhelming majority of swimming pools are treated with chlorine, this article will remain focused on chloramine vapor. It's the same basic idea, but bromamines are less corrosive and irritating to people.

 By
By