Swimming Pool Chlorine: 101
Swimming pools need a residual sanitizer. Chlorine is the most commonly used sanitizer in swimming pools around the world for many reasons. In this first article, we will discuss the what happens when chlorine is dissolved in water, the most common swimming pool chlorine products, and how chlorine kills and oxidizes contaminants.
Covered in this article:
- Chlorine's reactions with water
- Types of swimming pool chlorine
- Liquid Chlorine (sodium hypochlorite)
- Cal Hypo (calcium hypochlorite)
- Salt-generated chlorine (Cl2)
- Trichlor and Dichlor
- How chlorine kills
- How chlorine oxidizes
- Conclusion
Chlorine's reactions with water
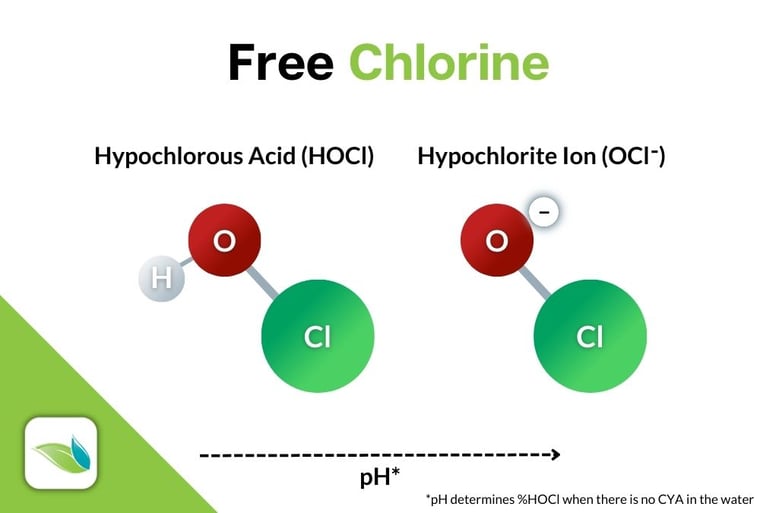
When any chlorine product is dissolved in water, specific chemical reactions occur to create the active killing form of chlorine, Hypochlorous Acid (HOCl), along with some byproducts. Each chlorine product has different byproducts, which we will elaborate on later in this article. For now, let's stay focused on HOCl.
When chlorine dissolves in water, the reaction creates HOCl and HCl, then HOCl separates into two forms of Free Chlorine (FC):
Cl2 + H2O → HOCl + HCl
Chlorine(gas) + Water → Hypochlorous acid + Hydrochloric acid
Then, a Hydrogen dissociation reaction occurs almost immediately within HOCl itself, creating the much weaker form of free chlorine, Hypochlorite ion (OCl-):
HOCl ⇌ H+ + OCl-
Hypochlorous acid ⇌ Hydrogen ion + Hypochlorite ion
This reaction can go back and forth based on pH, as long as there is no cyanuric acid (CYA) in the water.1 In other words, pH controls this equilibrium, and thus the strength of chlorine (%HOCl), but only in non-stabilized pools (like indoor pools). See the chart below.

The chart on the left is the most commonly-used representation of pH's impact on chlorine strength. The red line represents the powerful killing form of chlorine, hypochlorous acid. The blue line is its weaker counterpart, hypochlorite ion. If CYA is in the water, the chemistry fundamentally changes and it looks more like the chart on the right. Just look at the difference of the red line. Tap here to learn more about CYA's complicated relationship with chlorine and pH.
Since our website and services revolve solely around indoor swimming pools, let's stay focused on the left chart. Indoor pools do not use CYA because the water is not exposed to sunlight's UV rays (...usually).2 Because CYA is not used indoors, the pH directly controls the strength of chlorine.
Types of swimming pool chlorine
All chlorine types become HOCl and OCl-. Beyond that, each chlorine product has two different impacts on water: its byproducts left behind, and its temporary pH impact. So let's briefly look at each type of chlorine product and how it reacts in water.
Liquid Chlorine (sodium hypochlorite)

Chemical formula: NaClO (sodium hypochlorite)
pH: ~13
pH impact on water: Almost neutral, after chlorine oxidizes and kills contaminants. HOCl loses its oxygen and creates Hydrochloric acid (HCl), which almost completely neutralizes the high pH sodium hydroxide (NaOH) byproduct from liquid chlorine. So initially, the very high 13+ pH of liquid chlorine locally raises the pH of the water, but that is somewhat quickly reversed and almost neutralized once chlorine does its job.
Byproduct(s): Salt
Description: Liquid chlorine, aka bleach, is the most common form of chlorine used around the world. By itself it is neither flammable or explosive, but if mixed with other chlorine types (like cal hypo or trichlor, for example), an explosion can occur. Never mix different types of chlorine together. Of the primary chlorine types, liquid chlorine is the safest to handle, but it has a relatively short shelf life, due to its degradation rate. Household bleach is the same substance, but between 5-6% concentration, whereas swimming pool chlorine is between 10-12.5% concentration. The higher the concentration, the faster the degradation.
Cal hypo (calcium hypochlorite)
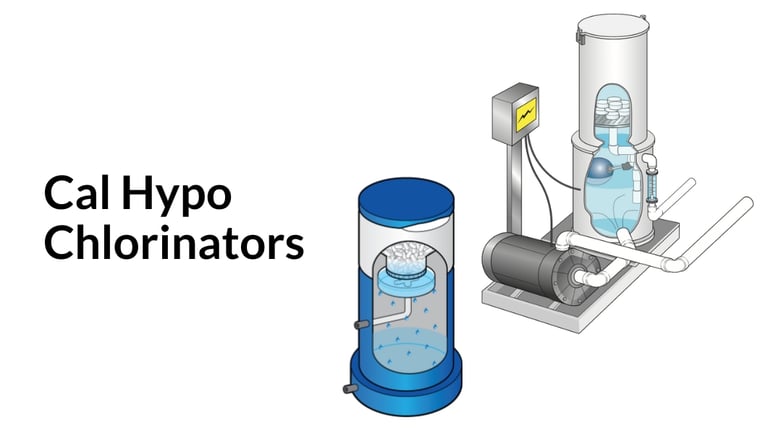
Chemical formula: Ca(ClO)2 (calcium hypochlorite)
pH: ~10.8
pH impact on water: Almost neutral, after chlorine oxidizes and kills contaminants. HOCl loses its oxygen and creates Hydrochloric acid (HCl), which almost completely neutralizes the high pH calcium hydroxide (Ca(OH)2) byproduct from cal hypo. So initially, the alkaline pH of cal hypo locally raises the pH of the water, but that is somewhat quickly reversed and almost neutralized once chlorine does its job.
Byproduct(s): Calcium, salt
Description: Cal hypo is a dry chlorine product that must be dissolved in water. In commercial swimming pools, cal hypo is almost always in pressed tab or briquette form, only to be added into specialized chlorinators. Due to its chemical nature, cal hypo is an incredibly powerful oxidizer, and that also makes it volatile. Always use safety gear and caution when handling cal hypo.
Salt-generated chlorine (Cl2)
Chemical formula: Cl2⇡ (site-generated chlorine gas)
pH: the chlorine gas is acidic (<1.0), but the byproduct NaOH is basic (>13).
pH impact on water: Salt chlorine generators are technically pH neutral, because the low-pH chlorine gas (Cl⇡) neutralizes the high-pH sodium hydroxide (NaOH). However, due to the turbulence created by Hydrogen gas bubbles (H2⇡), the pH rises in saltwater pools thanks to the loss of carbon dioxide (CO2).
Byproduct(s): Sodium hydroxide, hydrogen gas
Description: While not recommended for indoor pools,3 salt systems are popular in outdoor residential pools. When salt dissolves in water, the sodium (Na+) separates from the chloride (Cl-). The chloride is then charged by electricity in a process called electrolysis. This process creates chlorine gas (Cl2⇡), sodium hydroxide (NaOH), and Hydrogen gas (H2⇡). You can learn more about saltwater pools here, and go more in depth on saltwater chemistry here.
Trichlor and Dichlor (dichloro-s-triazinetrione)

Trichlor chemical formula: C3Cl3N3O3 (Trichloro-S-Triazinetrione)
Dichlor chemical formula: C3HCl2N3O3 (Dichloro-S-Triazinetrione)
pH: Trichlor is acidic (~2.8); Dichlor is more neutral (~6.5).
pH impact on water: Trichlor will lower the pH of water, and Dichlor will not make a noticeable impact on pH.
Byproduct(s): cyanuric acid, salt
Description: While not recommended for indoor pools, stabilized chlorines are popular in outdoor residential pools. Both dichlor and trichlor are manufactured with cyanuric acid. By weight, over half of each product is actually cyanuric acid, and the rest is chlorine. Since indoor pools do not have direct sunlight, these chlorines should not be used indoors. They should be viewed as cyanuric acid delivery products that happen to chlorinate, not the other way around. Too much CYA drastically slows chlorine and complicates the rest of water chemistry. Keep CYA to a minimum on outdoor pools, and don't use it indoors.
How chlorine kills
Chlorine is an exceptionally good disinfectant. It is known for its ability to kill a wide range of microorganisms, and in swimming pools, we need a residual sanitizer like chlorine to keep the water safe, clean and clear. While chlorine alternatives exist, like bromine, chlorine is by far and way the most commonly-used swimming pool sanitizer.
In fact, our own white blood cells produce HOCl as part of our immune system.3 The chemistry of chlorination killing germs can be simplified into just few characteristics.
- Active free chlorine (HOCl) is neutrally charged, which is attracted charged contaminants. Seek and destroy.
- HOCl disrupts cell membranes and penetrates into the cell, permanently damaging the cell wall or cell membrane (depending on the type of microorganism).
- HOCl oxidizes and destroys cellular components within the cell, which disrupts the DNA, preventing reproduction of the cell, and kills the cell itself.
How chlorine oxidizes
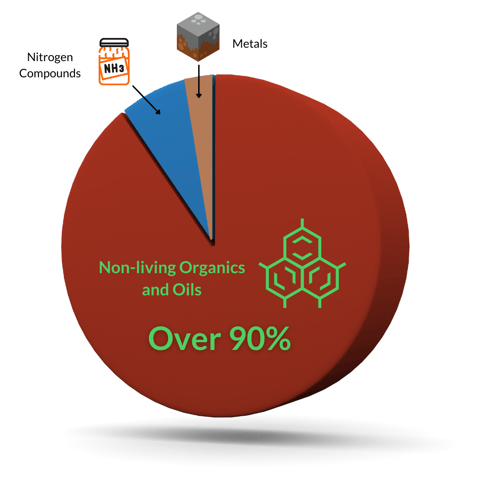
Chlorine kills living contaminants, but it oxidizes non-living contaminants. In swimming pools, there are three main categories of non-living oxidants:
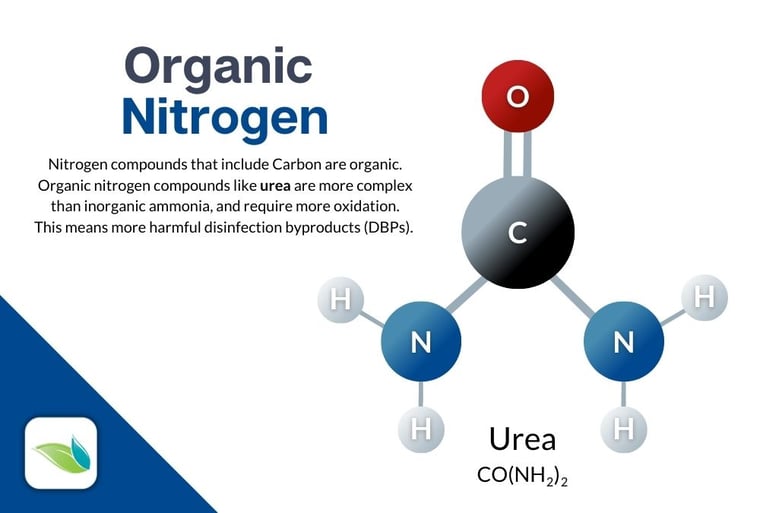
There are compounds that are both nitrogen-based and organic, such as Urea (CO(NH2)2). This means urea is more complex and takes more chlorine to fully destroy/oxidize. Urea is also one of the primary culprits for indoor air quality problems, because chlorine combining with urea creates many variations of chloramines and other disinfection byproducts (DBPs).
Oxidation-Reduction Potential (ORP)
Oxidation is a chemical process of stealing an electron from an oxidant, and in the case of chlorine, swapping an oxygen in the process. Reduction is actually the gain of an electron, which has a negative charge (e-), and therefore reduces the oxidizer.
We can measure water's potential for these reactions (which are called "redox" reactions) by measuring the conductivity of water, in millivolts (mV). This measurement is called Oxidation-Reduction Potential (ORP). Commercial swimming pool controllers almost always measure ORP, because it is a decent real-time indicator of chlorine's performance in water.
Many different oxidizers exist, but let's stay focused on chlorine. And for the purposes of this article. we'll keep this simplified.
Basically, HOCl steals an electron from an oxidant and replaces it with its Oxygen. This can be confusing because Iron (II) has two protons, and it becomes Iron (III) with three protons. Yet electrons are transferred from iron to HOCl. In this redox reaction, iron is oxidized (loses electrons) and HOCl is reduced (gains electrons that reduce its valence), resulting in Iron (III) and two byproducts: chlorides and water.
Here's the simplified reaction:
Fe2+ + 2HOCl → Fe3+ + 2Cl- + H2O
Iron (II) + Hypochlorous acid → Iron (III) + chloride + water
Water chemistry can be complicated, so we won't dwell on it. Just know that chlorine oxidizes non-living contaminants by stealing electrons from them, ultimately destroying them. This process reduces (or "uses up") chlorine, and creates various byproducts.4
Conclusion
Chlorine is the best residual sanitizer used in swimming pools. Chlorine is a strong disinfectant and decent oxidizer. Different chlorine products are available, and this article briefly covered each one. No matter which chlorine type is used, when chlorine dissolves in water, the active killing form of chlorine, Hypochlorous Acid (HOCl) is created. Each chlorine product has its own set of byproducts beyond that.
To keep water safely disinfected, a minimum of 1.0 ppm is recommended by health departments. However, it's really more about how much active HOCl is available. We'll cover more in a future article. We hope this has been helpful.
1 The relationship between pH and chlorine is direct when there is no CYA stabilizer in the water. The lower the pH, the higher the concentration of HOCl compared to the weaker OCl-. Therefore, in non-stabilized pools–like indoor swimming pools–the pH determines the strength and speed of chlorine. Lower pH means more effective chlorine. This is why health departments care about maintaining specific pH range. We need an effective residual disinfectant to keep water safe, clean, and clear.
2 There is an argument that can be made for using a very small amount of CYA in indoor pools, though the practice is not common. Read this white paper from renowned chemist Richard Falk. Usually, when we see indoor pools that contain CYA it's because the operators are using trichlor as a convenient chlorine product. We strongly recommend against using trichlor in indoor pools.
3 Andrés, C. M. C., Pérez de la Lastra, J. M., Juan, C. A., Plou, F. J., & Pérez-Lebeña, E. (2022). Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. International journal of molecular sciences, 23(18), 10735. https://doi.org/10.3390/ijms231810735
4 When nitrogen compounds and non-living organics are oxidized, these byproducts can be harmful. They are lumped into the broad category of "disinfection byproducts" (DBPs), commonly referred to generally as chloramines, even though chloramines are technically just chlorine oxidizing inorganic ammonia (NH3).

 By
By