Swimming Pool Disinfection Methods
Swimming pool water quality is about keeping the water clean, safe and healthy via disinfection. Therefore, disinfection is the top priority for swimming pool operations. So how is it accomplished?
Covered in this article:
Swimming Pool Chemistry- Water Quality
- Disinfection
- Oxidation
- Water clarity and cleanliness
- Ways to disinfect a swimming pool
- Residual sanitizers
- Secondary disinfection systems
- Conclusion
Swimming Pool Chemistry
In swimming pool chemistry, there are two disciplines: water quality and water balance. Water quality is about keeping the water clean, safe and healthy. It is the priority for swimming pool operations. Water balance is also important, but that is to keep the water itself happy and protect the pool and its equipment. So let's focus on water quality, and specifically disinfection.
Water Quality
Let's start by defining three terms:
-
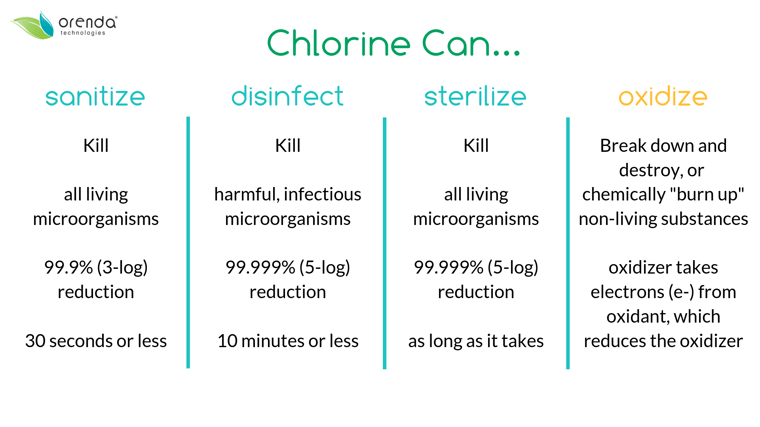
- Sanitization (or sanitizing): killing living microorganisms–like germs and algae–by 99.9% within 30 seconds (called a 3-log reduction).
- Disinfection (or disinfecting): killing harmful living organisms–germs, viruses, parasites, but not plants like algae–by 99.999% within 10 minutes (called a 5-log reduction).
- Oxidation (or oxidizing): destroying non-living contaminants by stealing electrons from the molecules and sometimes replacing them with oxygen.
Disinfection / Sanitization
The distinction between disinfection and sanitization is subtle. They are terms that describe a threshold that has been accomplisehd. And generally, they are accomplished at the same time using the same chemical(s) or secondary systems.
If you are achieving disinfection, you're most likely achieving sanitization too (and vice versa). Disinfection is a step further (99.999% reduction) than sanitization (99.9% reduction).
The difference is that sanitization also includes non-infectious microorganisms like algae. So, theoretically, you could achieve disinfection in the pool but still have some algae living, because algae can protect itself with a biofilm slime that acts as a shield. But usually, at least in the pool industry, these terms are used interchangeably.
We will elaborate on how disinfection is accomplished in a moment.
Oxidation
While santization kills living contaminants, oxidation destroys non-living contaminants. There are three main categories of oxidants: metals, nitrogen compounds, and non-living organics.
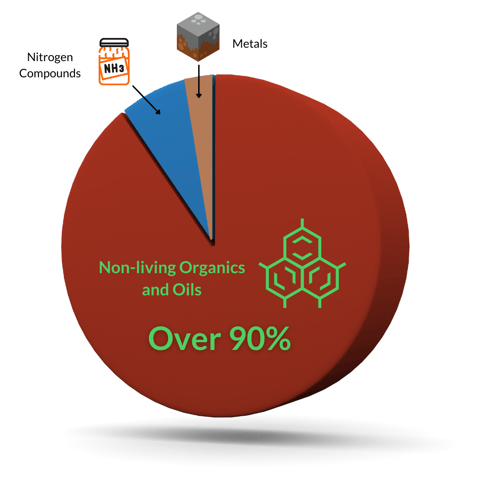 In most pools, metals are a very small percentage, if you have them at all. These heavy metals include iron, copper, and manganese.
In most pools, metals are a very small percentage, if you have them at all. These heavy metals include iron, copper, and manganese.
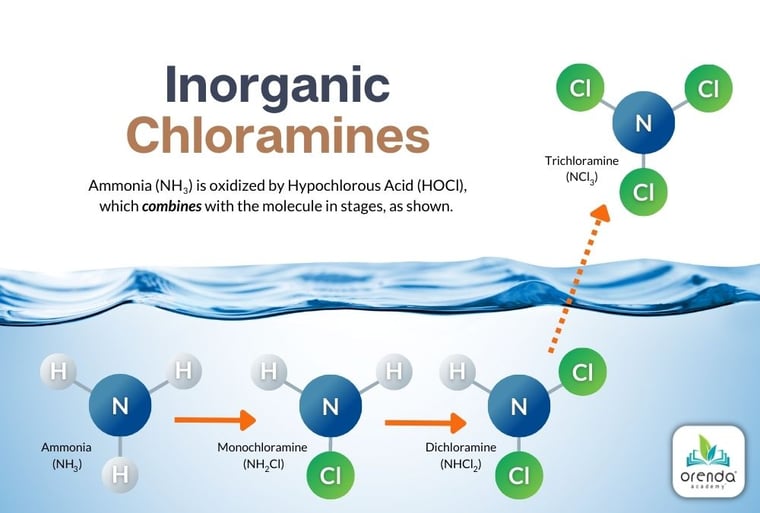
Nitrogen compounds include ammonia and urea, along with other various compounds that may also be organic (meaning they contain carbon too). The more complex the molecule, the more complex the oxidation process, and therefore, more chlorine is needed to oxidize it. Pure ammonia in a swimming pool creates inorganic chloramines at a 5:1 molar ratio of Hypochlorous acid (HOCl) to ammonia (NH3). Subsequent 5:1 molar ratios then yield dichloramine, and again to create trichloramine, which off-gases and is the main culprit behind the pool smell.
Related: Pool Chemistry part 2: Combined Chlorine
When organics are involved with nitrogen, the reactions are much more complex and require more chlorine to remove. Thankfully, enzymes are an excellent supplement to chlorine because they break down and remove these organics, leaving only nitrogen behind for chlorine to deal with.
Speaking of organics, non-living organics are the main type of oxidant demand in swimming pools. They are usually over 90% of the contamination in water, and they need to be managed accordingly. Chlorine alone tries, but fails to fully remove oils and organics. The result is scum lines on tiles, slimy and grimy filters, and increased chlorine demand. Here again, the way to manage these non-living organics is to supplement chlorine with enzymes and/or secondary oxidation systems like ozone or AOP.
Before and after using Orenda CV-600 enzymes
Ways to disinfect a swimming pool
Now let's get back to disinfection. There are two primary types of disinfection methods: residual sanitizers (usually chlorine) and secondary systems.
Residual sanitizers
The most common pool sanitizer, by far, is chlorine. Alternatives include bromine, and in a very small percentage of residential pools, biguanides and hydrogen peroxide can be used. In commercial pools, however, well over 90% of swimming pools use chlorine, and most of the rest use bromine. Both chlorine and bromine are halogen elements that dissolve in water to create powerful disinfection.
The byproducts of each sanitizer differ, but conceptually they are the same. For instance, when chlorine oxidizes ammonia it creates chloramines, whereas bromine oxidizing ammonia creates bromamines.
There are several types of chlorine products, but they all dissolve in water to create the strong, killing form of chlorine, Hypochlorous acid (HOCl); and its weaker counterpart, Hypochlorite ion (OCl-).
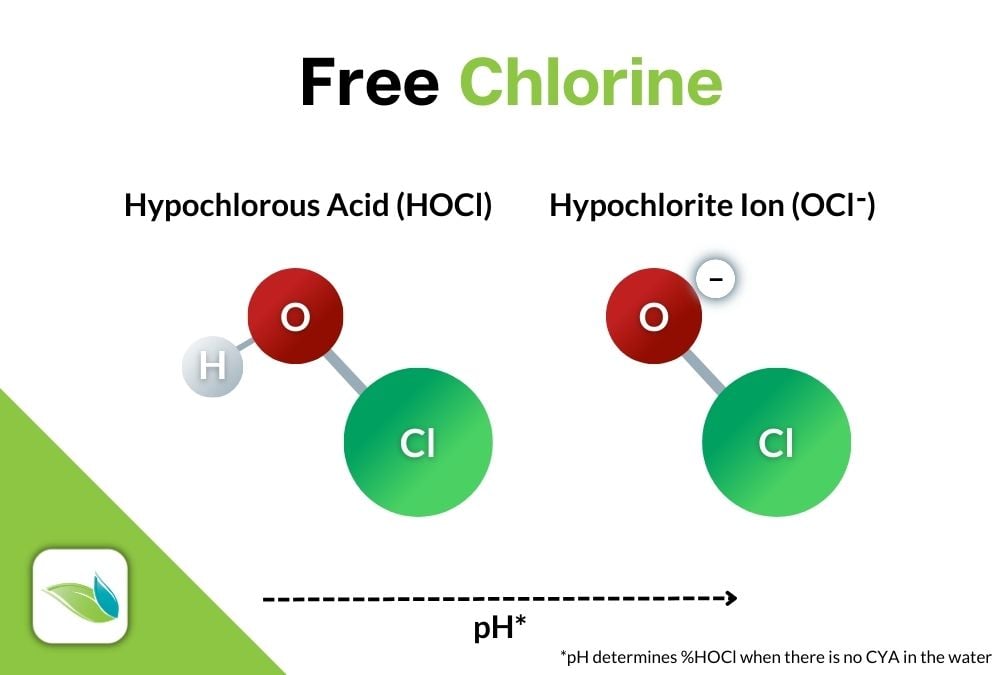
Cl2 + H2O → HOCl + HCl
Chlorine + Water → Hypochorous Acid + Hydrochloric Acid
Then a HOCl can dissociate into its weaker counterpart, OCl-.
HOCl ⇌ H+ + OCl-
Hypochlorous acid ⇌ Hydrogen ion + Hypochlorite ion
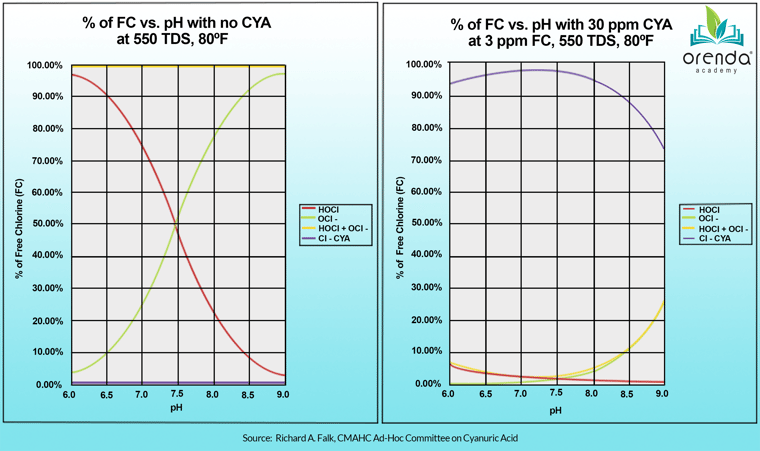
In water without cyanuric acid (CYA) stabilizer in it, the pH determines the percentage of HOCl vs. OCl-. See the chart below.
Types of chlorine include:
- Liquid chlorine (sodium hypochlorite)
- Cal hypo (calcium hypochlorite)
- Lithium hypo (lithium hypochlorite)
- Trichlor (Trichloro-S-Triazinetrione)
- Dichlor (Dichlor-S-Triazinetrione)
- Salt-generated chlorine (Cl2)
Lithium hypo is rare nowadays, and hardly ever used anymore. Trichlor and dichlor are stabilized chlorines that are popular for outdoor residential pools, but are not recommended for commercial pools as primary chlorine types. Salt systems are also common for residential pools, but less so for commercial, and we recommend against using saltwater systems for indoor pools.1
By far and away, the most common chlorine types for commercial pools are liquid chlorine and cal hypo.
Secondary disinfection systems
We believe every swimming pool needs a residual sanitizer in it as the first line of defense against harmful germs and other pathogens. But oftentimes chlorine or bromine can be overwhelmed with oxidants, and it needs help. That's where a secondary disinfection system comes into play.
Related: Comparing secondary disinfection systems for swimming pools
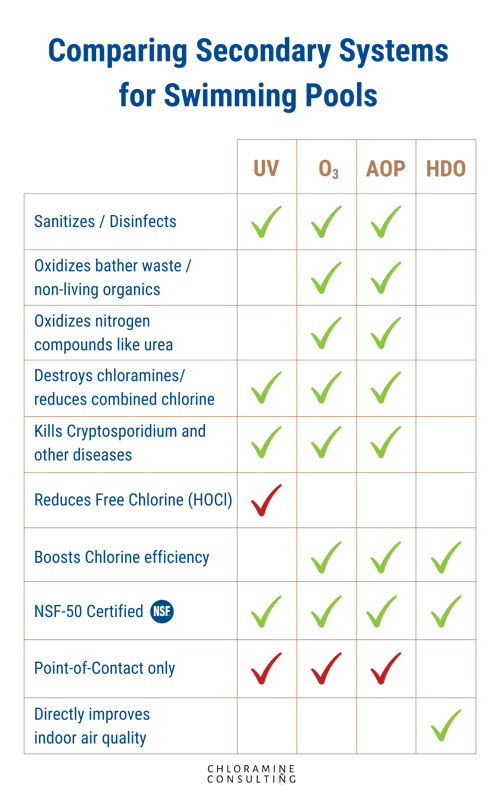
Secondary disinfection systems include:
- Ultraviolet (UV) systems
- low-pressure UV
- medium-pressure UV
- Ozone (O3)
- Advanced Oxidation Process (AOP)
These are point-of-contact systems that destroy just about anything that passes by them. Ozone and AOP are both disinfectants and oxidizers, which allow for a more broad scope of improvement to the water...but on indoor pools a contact tank/degassing chamber is required. UV, however, has 100% of the circulation flowing through its chamber where a bright UV light disrupts the cells of living microbes, rendering them dead and unable to reproduce.
Ozone and AOP destroy completely, while UV inactivates. Each of these systems, if installed correctly, can be highly effective at supplementing chlorine. One note about bromine, however: UV should never be used on a bromine pool. UV interacts with bromine to create harmful bromates. This is why bromine should not be used on outdoor pools either.
Conclusion
Water quality is all about keeping the water clean, safe, and healthy to swim in. Disinfection is arguably the most important function of pool maintenance. The most common residual sanitizer is chlorine, but bromine is also used sometimes. To ensure adequate disinfection in a busy commercial swimming pool, we recommend supplementing chlorine with enzymes and a secondary disinfection system.
1 Salt is not recommended for various reasons, but the main one is corrosion. Saltwater is inherently more corrosive than normal pool water, even if the pool has high TDS from years of chlorination with liquid chlorine or cal hypo. Salt pools need to be on a common electrical bond to a common ground, and often that is not the case. Galvanic corrosion is a major problem for saltwater pools, especially in pools with many metal components like ladders, bulkheads, stainless steel gutters and main drain covers.
Furthermore, salt systems create a constant amount of chlorine (depending on how they are programmed), which usually overchlorinate when the pool is not being used heavily, and under-chlorinate in times of heavy use. A more appropriate chlorination method is to feed liquid or cal hypo based on real-time demands like a free chlorine probe and/or ORP sensor. Modern chemical controllers have these features, but not all pools have such controllers.

 By
By