Molecular Weights of Airborne Chloramines in Indoor Swimming Pools
Chloramines and other airborne pollutants in indoor swimming pools behave differently than the rest of the air in the room. This is mostly because they are heavier than nitrogen (N2) and oxygen (O2), the two dominant gases in the air we breathe.
Covered in this article:
- What is a molecular weight?
- What is the molecular weight of chloramine?
- Comparing molar weights of Trihalomethanes and oxygen in indoor pools
- Effects of molecular weight on chloramine air dispersion and behavior
- Chloramines stay low in the room
- Airflow moves chloramines. Duct design matters!
What is a molecular weight?
Atoms are small. Very small. Way too small to handle individually. So in chemistry labs, experiments require enough material to be measured and manipulated. Such work requires vast numbers of atoms, which is made possible by using a special unit of measurement called the mole (or mol).

The mole bridges the gap between theoretical and practical chemistry, enabling us to touch and physically measure substances. Instead of trying to combine individual atoms together, we can combine one mole of each substance for practical experiments and calculations.
The mole, or Avagadro's Number, is a very large, yet precise amount of atoms: 6.02213076 x 1023. It is based on exactly how many carbon atoms are in twelve grams (12g) of Carbon-12, establishing that pure Carbon weighs 12 grams per mole (12 g/mol). Every other substance is measured using the exact same number of atoms (1 mol). Scientists can then physically weigh and handle that amount of material.
Nearly every element and substance has a known molar weight, and it is published online. For our purposes, this makes it easy to know the weight of a molecule compared to others.
In the context of indoor swimming pool air quality, let's compare the molecular weights of harmful airborne pollutants like nitrogen trichloride (trichloramine, NCl3), and cyanogen chloride (CNCl) with nitrogen (N2) and oxygen (O2).1
What is the molecular weight of chloramine?
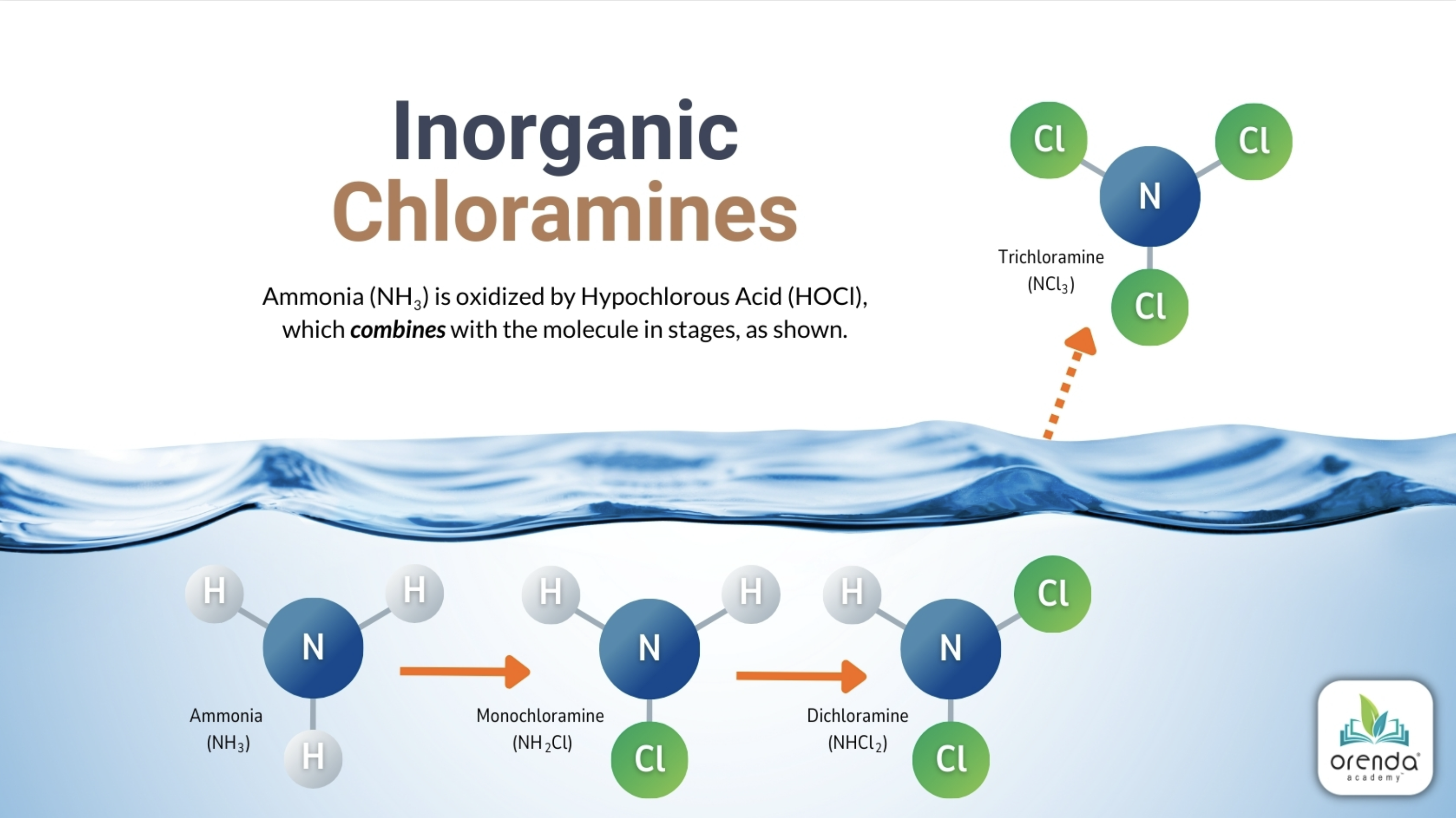
As mentioned in our other articles, the term "chloramines" is used to generically describe all the harmful disinfection byproducts (DBPs) that off-gas from a swimming pool into the air. Technically, chloramines describe inorganic monochloramine, dichloramine and trichloramine. Of these, mono- and dichloramines stay waterborne:

In the illustration above, trichloramine (or nitrogen trichloride, NCl3) off-gases into the air. The truth is, depending on the contaminants in the water and the amount of chlorine, time, temperature, and other factors, dozens upon dozens of variations of airborne pollutants will off-gas. Among them is cyanogen chloride (CNCl).
According to the National Institute for Occupational Safety and Health (NIOSH), cyanogen chloride's listed physical dangers include:
- Vapors may be heavier than air. They will spread along the ground and collect and stay in poorly-ventilated, low-lying, or confined areas (e.g., sewers, basements, and tanks)
- Hazardous concentrations may develop quickly in enclosed, poorly-ventilated, or low-lying areas. Keep out of these areas. Stay upwind.2
Just how heavy is cyanogen chloride? 61.47 g/mol.3
And nitrogen trichloride? 120.36 g/mol.4
So trichloramine is nearly twice the weight of cyanogen chloride...and NIOSH says cyanogen chloride vapors are heavier than air and spread along the ground. Based on their molar weights, it's clear that heavier trichloramine molecules behave the same way, only more so.
Comparing molar weights of Trihalomethanes and Oxygen in indoor pools

Trihalomethanes like chloroform (aka trichloromethane, CHCl3) are another type of airborne pollutant found in swimming pool environments. Like trichloramine, chloroform and other trihalomethanes are also heavier than oxygen and nitrogen.
The molar weight of trichloromethane (chloroform) is 119.37 g/mol.5
That's nearly four times heavier than the oxygen we breathe (O2): 31.999 g/mol.6 Believe it or not, most of the air we breathe is not oxygen. Approximately 80% of the air is actually nitrogen (N2), which is even lighter than Oxygen: 28.014 g/mol.7
As you can see, off-gassing chloramines in indoor pool facilities are several times heavier than oxygen and nitrogen.
Now let's look at how their heavy molar weights affect their behavior in an indoor pool.
Effects of molecular weight on chloramine air dispersion and behavior
There have been ongoing studies conducted by Dr. Ernest (Chip) Blatchley III and his team of graduate students at Purdue University. I had the privilege of speaking with him during a shared presentation at a swimming pool conference in 2024. What he showed the audience was fascinating. His team is modeling the behavior of airborne pollutants in swimming pools. Their central hypothesis is:
"With all other conditions being constant, the worst air quality in an indoor pool facility will occur during times of heaviest bather loading."
It seems obvious, but they have been doing many experiments to learn exactly what is happening. They want to identify (and quantify) the variables that impact indoor air quality so it can hopefully be predicted and problems can be prevented. Dr. Blatchley and his team's studies thus far show a close correlation between carbon dioxide and trichloramine behavior.
It makes sense to us. More people means more CO2 exhaled in the space; and more people in the water means more chloramines in the air.
It will be interesting to see the results of phase three of their research. Our hope is to better understand chloramines so the issues they cause can be eradicated worldwide. We will update this article when that information becomes available.8
Chloramines stay low in the room
Logic alone tells us these gases stay low in the room. But we have more than logic...we have evidence. We see plenty of cases of corrosion in countless natatoriums we have evaluated in person, where the corrosion is worse toward the floor, depending on the air circulation in the space. This indicates a higher concentration of chloramines at the floor level.
It is also backed up by swimmers' experience. Swimmers are breathing the worst of the air in the room, at the water surface. Coaches next, then lifeguards (sitting in an elevated chair). Look up "lifeguard lung" in our research section. Swimmers coughing during and after swimming is a symptom of this exact issue.
Other factors like air temperature, relative humidity (RH%), and air movement can impact chloramine behavior, but not their nature.
Airflow moves chloramines. Duct design matters!
We often see HVAC design flaws and mistakes in natatoriums when we are conducting in-person facility evaluations. After all, people do not hire us to visit pools with good air quality.
In nearly every natatorium we have personally visited and helped (over 350 since 2012), the HVAC was designed within the ASHRAE 62.1 standards. And yet, the air quality was abysmal. Why?
The issue centers on this: chloramines do not behave like the rest of the air in the space. They are heavier than air. Unlike heat and moisture (which rise and expand), chloramines stay low and dwell. How is a ceiling exhaust above the pool supposed to remove them? How is a high return intake going to remove them?
They won't, because they can't.

Supply and return airflow design are critical for indoor air quality. They are not the only things that matter, but they are the only things that are hard to change after the building is constructed. It's easy for a pool operator to improve a chemistry regiment, or for the cleaning staff to swap out floor cleaning chemicals that may be incompatible with pool water. But changing duct? That's not so easy. It's always more affordable to design for good air quality up front than to modify the building later.
Conclusion
We have other articles that explore the water chemistry involved in creating these chloramines and other byproducts. This article was to bring us to one undisputed fact: chloramine pollutants in natatoriums are heavier than the air we want to breathe, so they stay low in the room.
Sure, they can be stirred up and moved by supply airflow, as we discuss here. But generally speaking, swimmers, lifeguards, and anyone else spending time in indoor pools depend on mechanical engineers to design the air system to handle these pollutants. All to often, they are not even aware of the issue. Let's change that, together.
1 Of course, there are countless other variations of disinfection byproducts (DBPs) that off-gas from swimming pools, but you will find they are all heavier than the air we breathe.
2 National Institute of Occupational Safety and Health. (Retrieved June 2025). Cyanogen Chloride (CK): Systemic Agent. Section two, Emergency Response - Physical Dangers.
3 National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 10477, Cyanogen Chloride.
4 National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 61437, Nitrogen trichloride.
5 National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 6212, Chloroform.
6 National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 977, Oxygen.
7 National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 947, Nitrogen.
8 Dr. Blatchley and his team have been conducting this research for over ten years. He presented their latest findings during a class we were teaching together at the AOAP show in Reno, NV. The data charts are intricate and without Dr. Blatchley explaining them, they would do more to confuse this article than anything else. When the research is finally concluded and published, we will request permission to share it and cite it appropriately. Needless to say, I am excited to learn the results, because this is a big problem worldwide. A better understanding of chloramines can help us prevent them on a more widespread scale globally.

 By
By