Rust and Corrosion on Stainless Steel Pool Equipment
Stainless steel "stains less", but it is not stain proof. Indoor pool equipment is very often corroded by chloramines and moisture. Let's talk about why.
Covered in this article:
- Why does stainless steel rust?
- Choose cleaning products carefully
- Case studies of lethal corrosion in indoor swimming pools
- The hard costs of corrosion
- Why is corrosion so bad in indoor swimming pools?
- Conclusion
In this article, we are specifically talking about indoor swimming pool environments and why they often destroy metals. And not just in the natatorium itself; we are also talking about rampant corrosion in the pump room and chemical storage rooms. The answer boils down to two factors that accelerate corrosion, even on supposedly corrosion-resistant stainless steel: low pH, and high chlorine/chloride content in the air.
Why does stainless steel rust?

There are some brilliant metallurgists and material engineers out there, and some of them write articles that we have found online that are very helpful. When doing the research for this topic, we learned a lot that we did not know. We have hyperlinked to the sources we cite along the way.
Regular steel is made of iron and carbon. It is susceptible to rust and corrosion when it gets oxidized. The oxidized steel usually becomes ferric oxide (Fe2O3), which is reddish brown in color, and is the rust we are all used to seeing. Stainless steel, however, is different. Stainless steel is reinforced with between 12-30% chromium, and sometimes has some nickel and manganese. According to this source, chromium is what makes stainless steel resistant to corrosion and rust:
When stainless steel is exposed to oxygen, chromium oxide is created on the surface of the steel because chromium has a very strong affinity for oxygen. The chromium oxide is a very thin layer which doesn’t spall off, and it prevents further oxidation of the stainless steel. Even if stainless steel is scratched and the chromium oxide layer is removed, a new chromium oxide layer will form and protect the rest of the stainless steel beneath it. As long as there is sufficient chromium present, the chromium oxide layer will continue to protect the stainless steel and prevent it from rusting. - Alex Wensley, PSI
In other words, as long as there is at least 12% chromium exposed to the air, chromium oxide is the rust product, and it is small enough that it will not spall off. Normal steel's rust is ferric oxide, which has a larger molecular volume than the underlying iron atoms. Also from the same source cited above:
Ferric oxide doesn’t form a continuous layer on the steel because the oxide molecule has a larger volume than the underlying iron atoms, and eventually spalls off leaving fresh steel exposed which then starts a deleterious rusting cycle. - Alex Wensley, PSI
This is all well and good for most environments that stainless steel is used in. But indoor swimming pools have corrosive air pollutants, like chloramine vapor and trihalomethanes. Corrosion to stainless steel normally occurs when chlorine or chlorides are present, and nitrogen trichloride (aka trichloramine, NCl3) definitely fits that bill.
Choose cleaning products carefully
One common mistake we see with natatorium cleaning practices is using aggressive cleaning chemicals on stainless features. Household bleach-based and ammonia-based chemicals can accelerate the corrosion rate of stainless. Be sure to always use a cleaner that the stainless steel manufacturer approves. There are some great stainless steel cleaners out there.
Case studies of lethal corrosion in indoor swimming pools
In 1985, an indoor pool in Switzerland had a roof that tragically collapsed and killed 12 people. It was determined to be caused by "chloride-induced stress corrosion cracking" by the Federal Materials Testing Institutes of Duebendorf, Switzerland and Berlin, Germany:
The Federal Materials Testing Institute, based in Duebendorf, Switzerland, and the Federal Materials Research and Testing Institute of Berlin concluded that the collapse was the result of chloride-induced stress corrosion cracking. The steel rods had been pitted, causing the roof to cave in. - Corrosion Doctors
As if that were not sad enough, in 2011, a 5-month old baby was killed by a falling speaker that landed on her. The speaker's hanging brackets failed due to corrosion cracking too:
In November 2011 a 5 month old baby sadly got killed in a Dutch indoor swimming pool because 2 speakers and a speaker frame landed on her head. The speakers fell from 5 meters height after a stainless steel bolt was broken due to environmental cracking. Worldwide there have been many incidents, and several accidents, with cracking stainless steels in the swimming pool atmosphere. - NACE Int'l
The indoor air quality environment is not just about heat and humidity, it is also very much about the corrosivity of chloramines and other airborne DBPs. These DBPs accelerate corrosion in indoor swimming pools, destroying metal components all over the place. This corrosion is not just ugly and potentially dangerous–or even lethal, as we see in the cases above–it is expensive too.
Near misses
These fatal incidents are tragic, of course, but they are not as uncommon as you might think. There are many more incidents of near-misses that are thankfully not fatal. People get hurt, or if the facility is lucky, the damage occurs when nobody is in the room.
Supply ducts are heavy and can fall if fasteners and brackets are not corrosion-proof. Oftentimes metal duct will be suspended with galvanized or exposed steel components (rather than aluminum or epoxy-coated materials). Anything ferrous will oxidize easily in an indoor pool environment, leading to deleterious corrosion.
If you see rust and corrosion in the roof, support structures, duct or anything else supporting heavy objects above the pool and deck, report it to management.Take photos on your phone and email them so there is a paper trail with a date and time stamp. An ounce of prevention is worth a pound of cure. Otherwise it could be too late.
The hard costs of corrosion

Medium pressure UV system rusting in a commercial pool pump room
Replacing rail goods like ladders, lifeguard stands and starting blocks gets costly. These items can be thousands of dollars a piece, so it's important to keep them clean with stainless steel-friendly compounds that reinforce the protective layer. But if you think the metal components you can see around the pool are expensive, consider the costs of corrosion in a places only the facility staff see. Think of the pump room, which holds expensive equipment like UV systems, which are often stainless steel.

Or how about the pool dehumidifier itself? Recirculating chloramines is like chain smoking corrosive air. Replacing evaporator coils, compressors or other components in a Pool Dehumidification Unit (PDU) can cost tens of thousands of dollars.
The list goes on and on. Something as simple as screws or bolts will corrode and crack. It's not that the bolts themselves are expensive, but the labor and time required to replace them costs money. And in many cases, the screws and bolts are holding together (or supporting) very expensive equipment.
Roofs and structural steel is vulnerable too, though not as often as steel that is low in the natatorium. Why? Because airborne chloramines are heavier than oxygen, and they stay low in the room. This fact is what led to the invention of source-capture Evacuator® technology.
Related: Understanding Source-Capture Exhaust
Why is Corrosion so bad in indoor swimming pools?
What other types of indoor spaces do you know of that have such humidity, heat, and corrosive chlorides and chlorine byproducts in the air? What other types of indoor rooms contain such chemical content in the air? Apart from industrial plants and laboratories–which have safeguards and are built for the chemicals used–we cannot think of any. Corrosion is so bad in indoor pools because pools are not traditionally built to remove the chemical byproducts in the air.
Chloramine Consulting exists to help architects and engineers change that paradigm.
When airborne chloramines condense, the pH of the condensation is quite low, which drives much of the corrosion in and of itself. Metals tend to have a slightly colder surface temperature than other surfaces like concrete and wood. You probably already know this from personal experience...metal objects usually feel cold to the touch, even at room temperature.
Condensation, we believe, is the reason why most corrosion in indoor swimming pools looks almost like it was sprayed on, rather than poured on. There are clearly spots that rust before everything else. And we can also tell that rust is worse lower on the stainless steel than on higher parts, as mentioned before.

We do not know the exact pH of natatorium condensation, but we have tested condensation before. We had a pH that was lower than a pool test kit was capable of measuring, meaning it was below 6.5 pH. A client of ours had a more advanced pH meter and tested the pH of the water dripping from their PDU's coil. The condensation's pH was lower than 4.0.That is astounding. It was so low that the concrete around the unit was etched and the aggregate was exposed. That water is just condensed moisture from the air we breathe inside the natatorium. What do you think that air does to our lungs?
Related: Research Library - Health and Science Articles about Chloramines
For some context on pH, for those of you who are not into chemistry, pH is a logarithmic scale from 0-14, where every whole number is 10x greater or less than the whole numbers around it. 7 pH is perfectly neutral, which means 6 pH is 10x more acidic than 7, and 5 is 100x more acidic than 7 (because its 10x10). 4 pH is 1000x more acidic than neutral water. Here's a chart showing pH and some common pool chemicals:
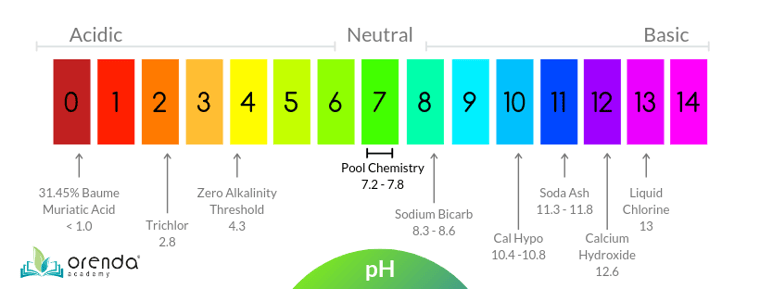
If you look all the way on the left side of this chart, you will see muriatic acid is below 1.0 pH. Is it any wonder why chemical storage rooms–when not vented properly and built with corrosion-proof materials–get such bad corrosion? There are too many corrosion photos to show, so we will create an image gallery and link to it in the near future. We have countless photos showing the brutality of swimming pool environments on metals. Like this one:

Conclusion
Indoor pool environments–and their pump rooms and chemical storage rooms–corrode metals rapidly. This is largely due to the high amounts of moisture in the air, which condenses on metals. The second factor is the high chemical content in the air; in particular, chlorine byproducts like chloramines, chloroform, and many other DBPs.
Stainless steel, despite being reinforced with chromium, still gets destroyed rapidly in a natatorium. This is not the fault of stainless steel, it's more a reality of an indoor pool environment that is not able to keep up with the air quality demands of the space. Cleaning stainless steel is mandatory, but do so only with stainless-steel friendly solvents and cleaners. Do NOT use ammonia-based or chlorine-based sanitizers like household cleaners. They will accelerate corrosion.
By and large, the best ways to mitigate and prevent rust around swimming pools is to have a properly- sized pool dehumidification system with well-designed airflow, and a source-capture exhaust system like the Paddock Evacuator® to efficiently remove the highest concentration of airborne chemicals in the room. Finally, good cleaning practices with the right cleaning products.
Believe it or not, water chemistry itself has less of an impact on stainless corrosion than you might think. Most of it is airborne condensation. While water chemistry does matter, it is less of a factor than climate control and removing corrosive chlorine byproducts in the air.
 By
By


