The Benefits of Indoor Pool Covers
Swimming pool covers are used for a variety of reasons on outdoor pools: safety, protection from the elements, and retaining heat. Indoor pools, however, use pool covers for one reason and one reason only: energy savings. This article will discuss how indoor pool covers impact not only energy consumption and indoor air quality, but water chemistry too.
Covered in this article:
- Types of indoor pool covers
- Safety Covers
- Solar/Thermal Covers
- Pool covers and indoor air quality (IAQ)
- Chloramine off-gassing
- Humidity Control
- Pool covers and energy savings
- Evaporation and heat loss
- Pool covers and water chemistry
- CO2 and pH
- Breakpoint chlorination
- Conclusion
Types of indoor pool covers
With outdoor pools, covers are usually just installed for the off-season to keep people, animals and natural debris from getting into the pool. These are called safety covers, and they serve an important purpose. But indoor pools–especially commercial pools–rarely have safety covers, because they are open year-round. Instead, they have bubble covers, or solar covers. Their purpose is to retain heat and minimize evaporation.
Pool safety covers
 If an indoor pool does have a safety cover, it's usually an automatic cover that rolls open along tracks. It makes no sense to have an anchored safety cover indoors, because again, indoor pools are usually open year-round. We won't spend too much time on this, we just mention it because these types of covers do exist, albeit rare indoors. (Image source: coverpools.com).
If an indoor pool does have a safety cover, it's usually an automatic cover that rolls open along tracks. It makes no sense to have an anchored safety cover indoors, because again, indoor pools are usually open year-round. We won't spend too much time on this, we just mention it because these types of covers do exist, albeit rare indoors. (Image source: coverpools.com).
Solar/Thermal covers
Yes, solar implies sunlight, but these covers are the most popular indoor covers too. They are typically a UV resistant plastic that is made specifically to withstand harsh chemicals, temperature ranges and direct sunlight. Indoors, however, these solar covers, also called bubble covers, are the most affordable and readily available option to cover the pool effectively. Theoretically, all an indoor pool needs is a vapor barrier, which could be as thin as plastic wrap from your kitchen. In reality, thin plastic is not practical for covering and uncovering the pool. So solar covers on large reels are used instead.
Solar covers are NOT safety covers. They just float on the surface of the water somewhat loosely, whereas safety covers are stretched tight and reinforced to support weight.
Pool covers and indoor air quality (IAQ)
An indoor pool cover will successfully thwart the off-gassing of chloramines, except in the areas that are not completely covered. Usually those areas are small, like corners, maybe a set of steps, or possibly gaps between two covers used in tandem. But by and large, a solar cover serves as a vapor barrier to drastically reduce–and virtually prevent–evaporation and chloramine off-gassing from occurring.
Chloramine off-gassing
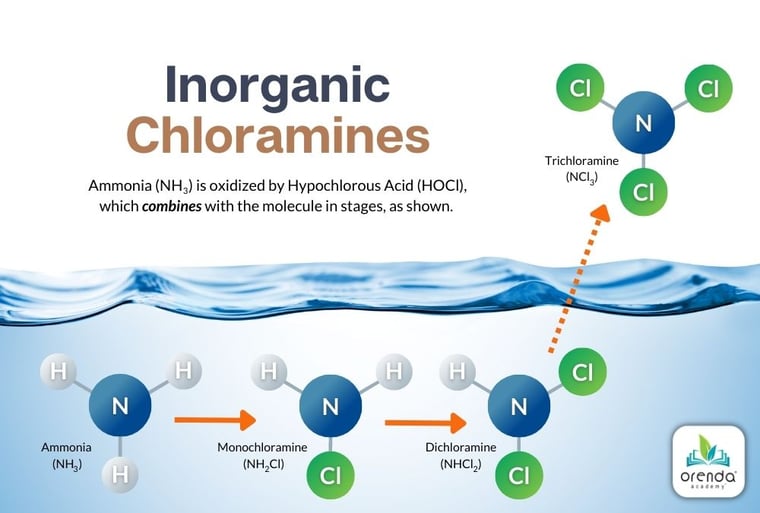
Remember the diagram above, from our other article explaining how disinfectant byproducts (DBPs) like chloramines are formed? You can see that part of the process for chloramine production is the final off-gassing of trichloramine, which is technically named nitrogen trichloride (NCl3). Some trichloramine remains in solution while some off-gasses, and thanks to physics, we know the rate of chloramine off-gassing can be accelerated by aeration (splashing, water features, etc.).
On the flip side of that, however, trichloramines are forced to stay in the water when the pool is covered. So when the pool is uncovered, pools usually get a surge of chloramine odor right away. So covers do not necessarily improve indoor air quality, they just push 'pause' on off-gassing...in a manner of speaking.
That being said, pools with secondary disinfection systems like UV or Ozone have more time to destroy trichloramine when it is held in solution. Once they off-gas, trichloramines become entirely an air issue.
Humidity Control
Indoor pools that are covered overnight tend to use significantly less energy on dehumidification because the moisture demand is reduced, therefore the pool dehumidifier uses less energy to maintain its set points. As we have mentioned in other articles, pool dehumidifiers are designed to maintain a relative humidity and temperature in the space, and they do so by removing a certain amount of moisture. If a pool cover is used, the amount of moisture in the air drops considerably, so the PDU does not need to use as much energy. That being said, the manufacturer of the PDU needs to know if a cover is going to be used in off hours, because that must be factored into their design.
In other words, covers reduce the workload for the PDU substantially, and reduce the amount of heat and humidity released from the pool into the natatorium.
Related: Pool Air Quality Resources
Pool covers and energy savings
Image source: Spectrum Aquatics
According to the US Department of Energy:
"Indoor pools aren't subjected to the environment, but they still can lose a lot of energy from evaporation. They even require room ventilation to control indoor humidity caused by the large amount of evaporation. The ventilated air also must be conditioned, which adds to the energy costs.
Pool covers minimize evaporation from both outdoor and indoor pools. Covering a pool when it is not in use is the single most effective means of reducing pool heating costs. Savings of 50%–70% are possible.
Pool covers on indoor pools not only can reduce evaporation but also the need to ventilate indoor air and replace it with unconditioned outdoor air. You can also shut off exhaust fans when an indoor pool is covered, which saves even more energy."
Evaporation and heat loss
50-70% savings on energy costs?! Yes, you read that correctly. The vast majority of heat loss is from evaporation. Here's a specific example of what me mean, also from that same page on the US Dept. of Energy website:
"Swimming pools lose energy in a variety of ways, but evaporation is by far the largest source of energy loss. Evaporating water requires tremendous amounts of energy. It only takes 1 Btu (British thermal unit) to raise 1 pound of water 1 degree, but each pound of 80ºF water that evaporates takes a whopping 1,048 Btu of heat out of the pool."
A pool does not need a thick cover to minimize evaporation loss, it just needs a vapor barrier. So beyond reducing humidity and chloramine off-gassing, pool covers save energy. They also impact water chemistry in a surprisingly big way.
Pool covers and water chemistry
There are a few aspects to water chemistry that pool covers impact, but we want to focus on two of them: pH and breakpoint chlorination.
CO2 and pH
Most people are unaware that water's pH is determined by the amount of carbon dioxide (CO2) dissolved in water. The more CO2, the lower the pH, and vice versa. Since water always seeks equilibrium, CO2 leaves the pool (off-gasses) to equalize with the CO2 in the air. This is called Henry's Law of the solubility of gases. We happen to know that the percentage of CO2 in the air is pretty low, and it leads to a pool pH rising to about 8.2, depending on the water's level of carbonate alkalinity.
Pool covers prevent this off-gassing of CO2, and therefore the natural pH rise does not occur as normal until the pool is uncovered again. We say "as normal" because there is still some aeration in the gutters and surge tank, but it's a fraction of the surface area of the pool, and somewhat negligible. So pool covers suppress pH, which can reduce the acid and/or CO2 demand in the pool. As a result it can also reduce the sodium bicarbonate demand to offset what acid would otherwise reduce.
By suppressing pH from its natural rise, in a non-stabilized pool, lower pH means stronger chlorine (higher %HOCl).1 While this may produce chloramines faster, higher %HOCl also disinfects more effectively, and that's a good thing for the health and safety of the pool.
Breakpoint chlorination
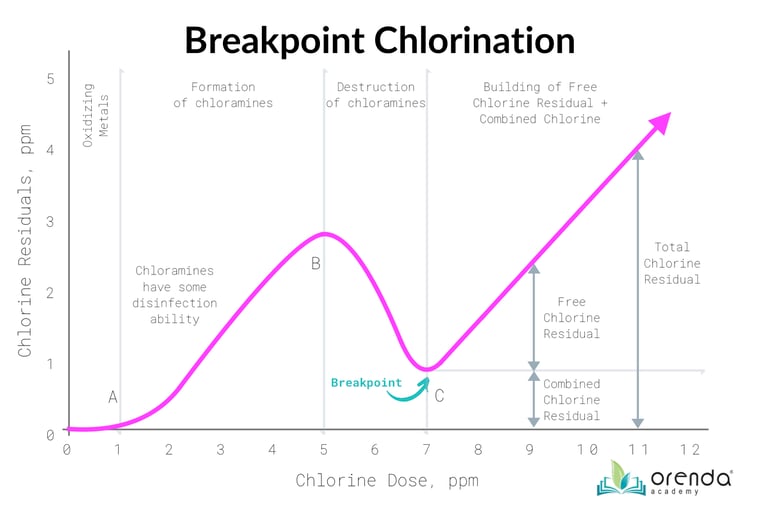 We mentioned earlier that covering a pool keeps trichloramines in the water longer, preventing them from off-gassing until the pool is uncovered. This impacts the breakpoint chlorination process because it gives chlorine more time to oxidize. It also gives secondary systems more time to destroy trichloramine. Trichloramines need to either off-gas out of the pool, or be destroyed by ozone or UV. Chlorine itself has already oxidized ammonia as far as it can (see the graphic above).
We mentioned earlier that covering a pool keeps trichloramines in the water longer, preventing them from off-gassing until the pool is uncovered. This impacts the breakpoint chlorination process because it gives chlorine more time to oxidize. It also gives secondary systems more time to destroy trichloramine. Trichloramines need to either off-gas out of the pool, or be destroyed by ozone or UV. Chlorine itself has already oxidized ammonia as far as it can (see the graphic above).
Not everything about pool covers is beneficial, however. If the covered pool already has too much combined chlorine, the cover will not help...it will only prolong the issue. Off-gassing trichloramine is an essential part of the breakpoint chlorination process.
Conclusion
Using indoor pool covers are very beneficial for energy savings, water savings2, and to a lesser degree, chlorine strength and pH control. But there are some drawbacks to covers too, depending on how you look at it. Breakpoint chlorination can take longer because chloramine off-gassing is suppressed. This can be a disadvantage or an advantage, depending on the systems you have in place in your pool. If you have a secondary disinfection system that can destroy chloramines, it could be an advantage because trichloramines are forced to stay in the water longer. If not, it's a disadvantage and your pool may struggle to stay on top of combined chlorine levels.
We hope this article has shared the pros and cons of indoor pool covers. If you have more questions, contact us or comment below. Thank you.
1 Non-stabilized means the pool contains no Cyanuric Acid (CYA). CYA binds with chlorine and negates the control that pH would otherwise have on the % of HOCL (the strong form of chlorine, hypochlorous acid).
2 Water savings happen because less water evaporates from the pool. This evaporation loss could be minimized by recycling that water from the dehumidifier's evaporator coil.

 By
By



