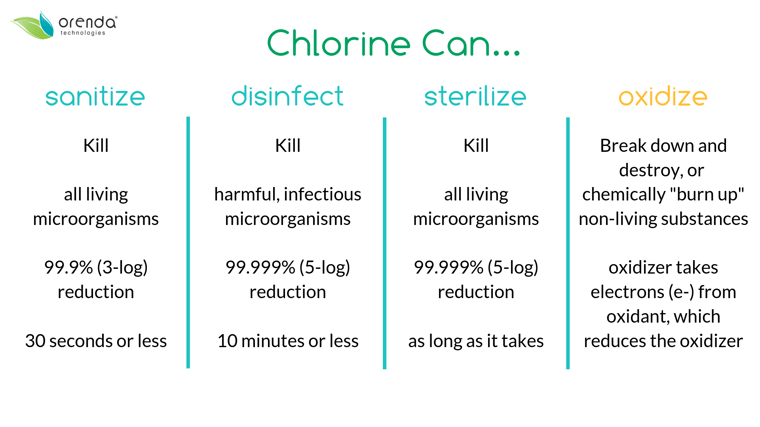
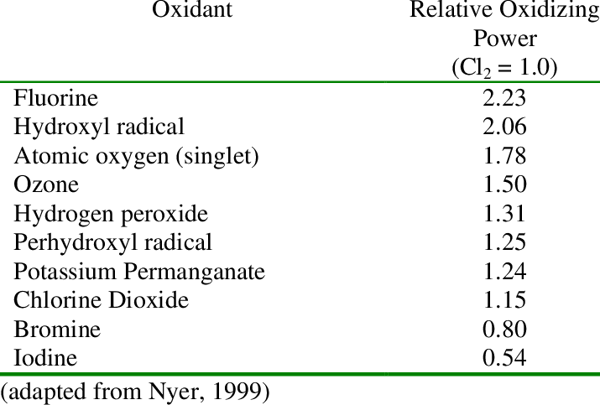
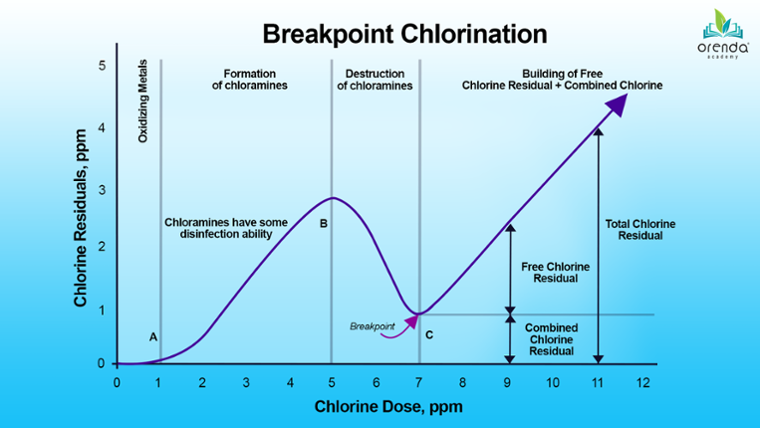
Nitrogen, however, is much harder to remove. Chlorine combines with nitrogen compounds like ammonia, nitrates and others to eventually break them down and off-gas byproducts into the air. It's nasty stuff, and nitrogen compounds are the root cause of chloramines. Chloramines (monochloramine, dichloramine and trichloramine) are just three of several hundred types of disinfection byproducts (DBPs) that are formed when chlorine attacks nitrogen.
The most common form of nitrogen in this context is ammonia, the primary component of urea, which is found in our urine and sweat.
Related: How to Reduce Combined Chlorine
Secondary Systems
Chlorine could use some help in high-use swimming pools. Secondary systems like Ultraviolet (UV) and Ozone are common. UV deactivates contaminants including some combined chloro-organics like monochloramine. Ozone is a secondary disinfectant and powerful oxidizer that destroys just about anything. Another secondary system is Advanced Oxidation Process (AOP), which combines UV and Ozone to create an even stronger oxidizer, hydroxyl radicals. (•OH).
All of these things are beneficial in managing water chemistry and combined chlorine. None of them, however, create a long lasting residual that can really impact things out in the swimming pool. They are point-of-contact systems at the mercy of circulation. These systems can only impact the water that flows through them.
Recently, a new secondary system has shown a lot of promise, and it is called Hyper-Dissolved Oxygen (HDO). HDO is dissolved into water as nano-bubbles, and can create a residual out in the pool itself. Without being certain of the exact science behind what is going on, it appears that high levels of oxygen help water quality and clarity. Could it be that this hyper-dissolved oxygen is helping chlorine out in the pool? Is it assisting in oxidation? We don't know, but this system's impact on indoor air quality is powerful, and undeniable. If you have indoor air quality concerns, it's probably a combination of water chemistry and air handling design. We can visit your facility and help diagnose it accurately.
Summary
Contaminants that are living organisms need to be killed, via sanitization/disinfection. Non-living contaminants like carbon and nitrogen-based bather waste need to be oxidized. Chlorine is often overwhelmed by non-living oxidants, which interferes in its ability to kill living pathogens. Is your pool relying solely on chlorine to stay clean of all contamination?
If so, consider secondary systems to help, and/or enzymes to remove non-living organic waste. These things can dramatically improve chlorine efficiency and keep your water cleaner. In Part 2, we will discuss nitrogen compounds more in depth.
1 Lowry, R. (2018). Pool Chemistry for Residential Pools. Pool Chemistry Training Institute, (pg. 115).

 By
By