5 Misunderstandings about swimming pool pH
Of all water chemistry parameters, many experts argue that pH is the most important. Indeed, pH impacts just about everything else in water chemistry, but most swimming pool operators–both residentially and commercially–misunderstand it. We know this because we also misunderstood it, thanks to reading swimming pool operator textbooks. This article will set the record straight.
Covered in this article:
5. pH and bather comfort4. How does alkalinity relate to pH?
3. pH control: is it even possible?
2. pH and chlorine strength
1. pH and carbon dioxide
5. pH and bather comfort
Why are we told to maintain pool pH between 7.2 to 7.8, and ideally 7.4 to 7.6? According to the textbooks and online resources, three reasons are cited.
- First and foremost, pH controls the strength of chlorine (%HOCl), which is legitimate for indoor pools. This directly impacts contact times (CT values) for disinfection. We will elaborate on this later in this article.
- The second reason is bather comfort, and
- The third is to protect pool surfaces and equipment from corrosion/etching and scale formation.
Indeed, pH impacts almost all other chemistry in swimming pools. But is it the only thing that drives these three circumstances? Not at all. All three of these are valid concerns, but pH itself is only partially responsible (if at all). Of these three, one that matters a lot to us is bather comfort, given that we have many years of swimming experience ourselves.
The pH of our eyes
When speaking about ideal pool pH levels, most pool professionals mention the pH of human tears, or the pH of our eyes. According to one scientific study, the pH of our eyes is usually 7.11 ± 1.5.1 That is a substantial pH range! Depending on how hydrated we are, that means the pH of our eyes can range between 5.61 and 8.61. Other ocular studies have shown similar results, but with a tighter pH window between 6.5 and 7.6.2
It seems clear that there are variables that affect the pH of our tears, but let's not get lost in the weeds. The real question, as it relates to swimming pool pH, is whether or not a pool's pH is what irritates our eyes or skin. If our eyes burn, is it from the pH? Or something else? And either way, how do we know for sure? How can we prove or disprove it?
In our experience, irritation of eyes and overall bather comfort has less to do with the pH of the pool, and more to do with chloramines and other harmful byproducts like trihalomethanes (THMs).
The byproducts of chlorine oxidizing various bather wastes is known to irritate people, whether it be off-gassing into the air or not. Itchy, dry skin after swimming is a common consequence of either too much chlorine in the water, or too many harmful disinfection byproducts (DBPs).
Let's be clear: we do not doubt that if pH is way low or way high that it can irritate bathers, because that should be obvious. The question is whether or not bather comfort is even noticeable within the pH parameters often seen in swimming pools. To illustrate our point, let's expand the pH extremes out to 6.2-8.8 pH, for a worst case scenario discussion. Hopefully, your pool will not hit either of these extremes, but with overcorrections and etching plaster, these numbers are definitely possible in swimming pools.
What will 6.2 pH water feel like? How about 8.8, or even into the 9's? Fortunately we don't have to wonder, because we drink bottled water all across this range of pH, and even wider. Want to know what ~6.0 pH water feels like? You've probably tasted it and it was refreshing. What about alkaline water? Many of us actually pay extra for high-pH bottled water to drink.
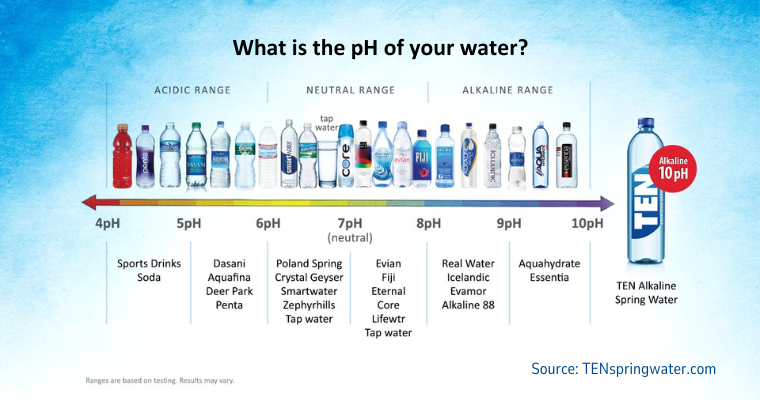
The point is, we drink bottled water across a wide range of pH levels. For instance, bottled water with a 5.5 pH is ten-thousand times (10,000x) more acidic than alkaline bottled water with a 9.5 pH. Yet both taste fine and we usually don't notice the difference. These pH's are far outside the bounds of what a normal swimming pool pH would be. So is it really the pH driving bather discomfort?
Maybe. But we doubt it. Some European standards (like the German DIN standard) encourage a pH between 6.5 and 7.0. From our own personal experience swimming in German and Italian pools, those pools feel great. Partially because they limit the free chlorine levels to 0.6 mg/L (ppm).3
4. How does alkalinity relate to pH?

Can anyone truly control alkalinity and pH changes? If so, please have them contact us...because it's physically impossible. The best we can do is leverage physics to contain pH using alkalinity as the mechanism for doing so. We'll elaborate on containing pH in our next section.
While pool operators can add a measured amount of sodium bicarbonate to set a Total Alkalinity (TA) level, the TA will fluctuate over time. TA is lowered every time acid is added–or in the case of pools using carbon dioxide (CO2) injectors, the alkalinity tends to increase over time.4 The point is, both pH and alkalinity levels move. This is a far more complex subject than we have time to discuss in this article, but suffice it to say, understanding alkalinity's relationship with pH will help us keep water chemistry balanced.
Alkalinity is a pH buffer
Most pool operators know that alkalinity "buffers the pH". But what does that mean? Technically, alkalinity buffers (slows) the change in pH because it can both give and receive a Hydrogen (H+) ion. To make sense of this, we first need to understand what pH is. The pH is the potential of Hydrogen (potenz Hydrogen, or also called the power of Hydrogen). It's all based on how water interacts with substances dissolved in it. Here's an infographic:
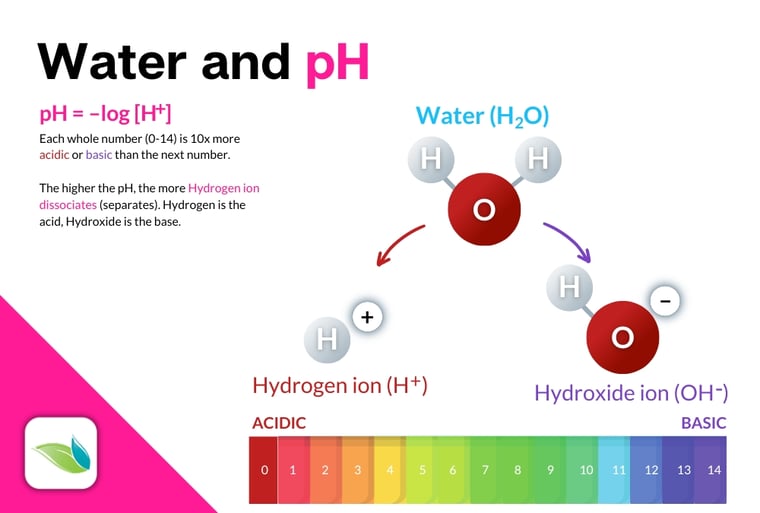
The higher the concentration of Hydrogen ions (H+) connected to a dissolved substance, the more acidic it is. The opposite is also true; as Hydrogen ions break away from dissolved substances, they become more basic, or alkaline. The pH goes up.
When Hydrogen ions move around, so too does the pH of a given solution. Alkalinity ions like bicarbonate (HCO3-) have the ability to bind to a Hydrogen ion, and they can also release it once again. This means alkalinity ions neutralize acids, which slows the change in pH. Hence, alkalinity is called a buffer.
Here's another infographic that shows how pH determines the percentage and type of alkalinity species found in most swimming pools. This is the carbonate alkalinity equilibrium, also called the carbonic acid equilibrium:5
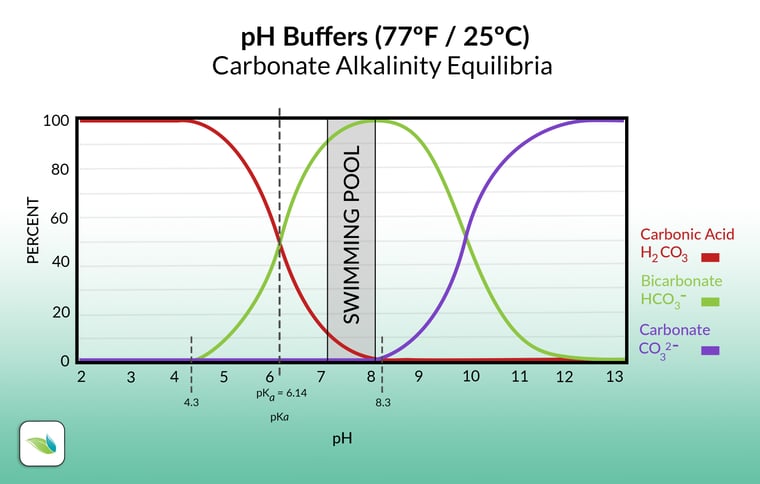
The chart above shows various forms of carbonates in water. On the far left, you have carbonic acid (H2CO3), which is just dissolved carbon dioxide:
CO2 + H2O ⇌ H2CO3
carbon dioxide + water ⇌ carbonic acid
As the pH rises, one Hydrogen leaves, and a new species of alkalinity emerges. Carbonic acid converts into its conjugate base, bicarbonate alkalinity (HCO3-). This functions in equilibrium, so the two substances always add up to 100%. Then, once the pH gets to 8.3, there is no more carbonic acid. Another Hydrogen leaves and another species of alkalinity presents itself. Bicarbonate converts into carbonate alkalinity (CO32-). The 8.3 pH threshold is important for water balance reasons, which we can explain in a future article.
The amount of alkalinity determines how much acid is needed to reduce the pH a given amount. The more TA, the more acid required to do the same pH reduction. A given pH reduction in a pool with 60 ppm TA will require about half the acid of that same pool if it had 120 ppm TA.
But alkalinity is not only important for buffering against a drop in pH...it is also a source of rising pH.
Alkalinity determines how carbonated the water is
Most of total alkalinity is bicarbonate ions, assuming the pool's pH is between 6.14 and 8.3. There are more types of alkalinity that are not even on the carbonate spectrum: cyanurate alkalinity (if you have CYA in your pool), and borate (if you use borates) also contribute to TA.
What matters for water balance (using the Langelier Saturation Index, or LSI) is the carbonate alkalinity, also called the corrected alkalinity when doing the LSI formula. The carbonate alkalinity is important for another reason beyond the LSI formula: the carbonate alkalinity determines how high the pH will naturally rise, because it determines how carbonated our water is. And that brings us to our next point.
3. pH control: is it even possible?

Given the technology available for commercial and residential swimming pools, one could be justified for thinking pH can be controlled. But pH cannot be controlled. The best we can do–without utilizing physics to our advantage–is use technology and chemicals to temporarily suppress pH from naturally rising too much.
Sure, with enough acid and other adjustment chemicals, you can force water's pH to stay within a certain range (say, 7.4 to 7.6), but doing so forces water into an unnatural position where it cannot stay.
Here's an analogy: an airplane can defeat gravity...temporarily. But one way or another, the plane will come back down. Flying requires a greater force than gravity, which expends finite energy.
In the same way, pH can be suppressed with acid or CO2 injection, but it will not stay there. Thanks to natural physics, the pH will rise due to the loss of CO2, because gases in water will equalize with those same gases above the water (Henry's Law).
Our chemicals and technology can only temporarily suppress pH from its natural rise toward equilibrium with the air above the water.
Remember that pH constantly fluctuates–much to the frustration of pool operators worldwide. This frustration is understandable, but that doesn't change the fact that physics control pH, and we cannot. All we can do is manipulate it temporarily.
Since the carbonate alkalinity ions in our swimming pools absorb and distribute Hydrogen ions (buffering pH), there's another way we can think about pH control. In pools with carbonate alkalinity, the amount of dissolved CO2 determines the pH.6 More on this later in this article.
So what should we do, because we know pH must be kept within certain ranges for optimal chlorine strength.7 The answer: use physics to our advantage first, then supplement with technology to limit the natural pH rise.
pH control? Or pH suppression?
In the aquatics industry, "pH control" really means pH suppression. Since the pH will naturally rise up to its equilibrium point, we prefer to think of pH management in terms of containing pH rather than trying to control it.
pH suppression is usually done with a chemical controller that has a pH probe, and the ability to feed acid or CO2 injection as needed. These systems are great, but like anything else, they have their limitations. Acid doses depend on TA levels, which cannot be digitally read in real-time. This means the chemical controllers can quickly deplete alkalinity if the pool chemistry is not regularly monitored by a qualified operator.
CO2 injectors are often preferred, especially on indoor pools, because they do not reduce the TA level.4 Operators can maintain a lower TA level, which reduces the rate of pH rise, and therefore maintain excellent water chemistry with less chemical costs.
2. pH controls chlorine strength?
Chlorine strength is defined by the percentage of active chlorine, Hypochlorous acid (%HOCl) compared to its slower-and-weaker counterpart, Hypochlorite ion (OCl-). The top priority pool operators is adequate disinfection and oxidation in pools. This is the essence of water quality, and disinfection contact times are the main reason health codes limit the pH from exceeding 7.8 in commercial swimming pools in the United States. See the chart on the left below.
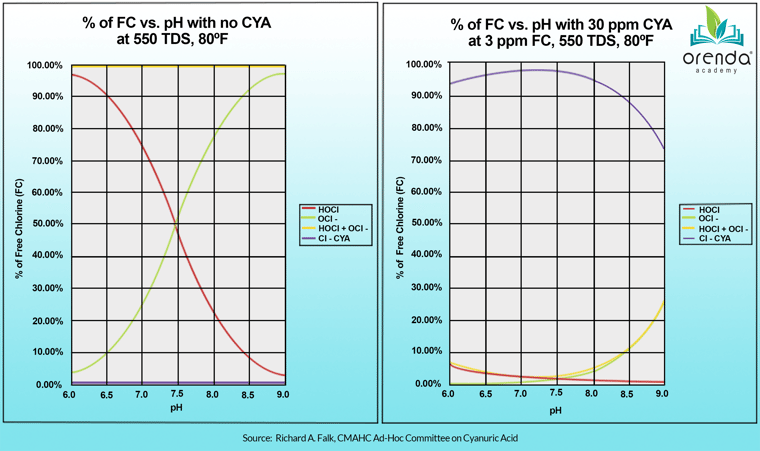
As you can see on the left, the lower the pH, the higher the percentage of the red line, HOCl. This translates to stronger and faster chlorine, which optimizes disinfection. And since this website is devoted to air and water quality for indoor pools, this chart is meaningful.
But this is for pools with zero cyanuric acid (CYA) in the water. This is a very important distinction to make, because outdoor pools that have CYA stabilizer in them cannot use that chart. Their chart looks more like the one on the right, which is based on 30 ppm CYA. The higher the CYA, the flatter the red line gets at the bottom.
It's not to say that pH doesn't still impact outdoor stabilized pools. It's saying that the pH does not control the strength of chlorine anymore....the ratio of free chlorine to CYA (FC:CYA) does.7 Just look at the red line on the right chart. Can you tell the difference in %HOCl from 7.0 to 8.0? or even 8.5?
That being said, when pH gets above about 8.3, the weaker form of chlorine, Hypochlorite ion (OCl-) dissociates from CYA. It breaks away, as you can see on the right chart toward the bottom corner. When it separates from CYA, it can be destroyed by sunlight. So as the pH rises pas 7.5, an increasing proportion of free chlorine can break away and be lost to sunlight degradation because it leaves CYA's UV protection. In practice, this chlorine loss is generally not noticeable until about a pH of 8.3.
The bottom line: indoor pools without any CYA in them must manage the pH and suppress it from rising too high, because lower pH levels optimize chlorine for disinfection. Outdoor pools with CYA do not have this issue. So pH does not always control the strength of chlorine.
1. pH and carbon dioxide (CO2)

And now, for the final topic of this long, detailed article. pH is a negative logarithm of Hydrogen ions (H+). The lower the pH, the higher the concentration of Hydrogen, and vice versa. But for practical purposes, that definition is hard for us to visualize, especially in a pool. After all, we're not chemists. Thankfully there's a much easier way to think about pH.
In pools with carbonate alkalinity, the amount of dissolved carbon dioxide (CO2) determines the pH of water.
The more CO2 in solution, the lower the pH, and vice versa. And since pools are over-carbonated compared to the air above them (thanks to carbonate alkalinity) that CO2 needs to equalize with the air above the swimming pool. This equalization is explained by a law of physics called Henry's Law.
Just like when you crack open a beer, CO2 escapes because it is trying to equalize with the air above the beer. Eventually enough bubbles make it to the top and the beer goes flat. Voila, Henry's Law.
To reference back to our earlier point, containing pH is not only possible, but easier, more cost effective, and more practical. Here's what we mean:
pH will naturally rise over time due to the off-gassing of carbon dioxide (CO2). This is normal, thanks to Henry's Law. When CO2 reaches equilibrium with the air above the pool, it goes "flat", like a soda or beer. No more CO2 can be lost, so pH can no longer rise.
Containing pH means building a foundation with the Langelier Saturation Index (LSI) as your floor, and pH(eq) as the pH ceiling. Reducing carbonate alkalinity will lower this ceiling according to the chart below.
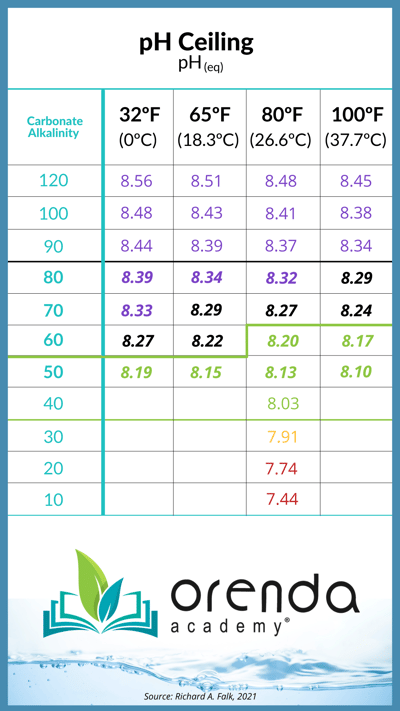
We alluded earlier when we were discussing alkalinity that alkalinity plays an important role not only in buffering pH, but in lowering it. This sounds counterintuitive, but it's the truth. In order to lower pH, you must have more CO2 in the water. But acid, like muriatic (HCl) contains no CO2. So where does it come from? Refer back to the chart earlier, and you'll see that acid simply converts carbonate and bicarbonate alkalinity down into carbonic acid, which brings down the pH. In simpler and not-so-scientific terms, acid burns through alkalinity to lower the pH. This is why they both get reduced with acid, and why CO2 injection only lowers pH, not alkalinity. CO2 injection bypasses this process.
Let's stop chasing pH, and embrace physics. Yes, pH is going to rise, but the chart above shows us that we can determine how high it can go. Aim to control your chemistry accordingly.
You can use the Orenda App to figure out your pH ceiling easily. Simply use the calculator and select "show" secondary readings. The pH ceiling appears in real-time as you adjust your chemistry dials.
Conclusion
To recap: pH is important in water chemistry, but not necessarily for the reasons we might think.
- First, we don't buy the bather comfort argument, because in our experience it's really disinfection byproducts like chloramines that irritate swimmers.
- Second, pH controls the type and percentage of each species of carbonate alkalinity, and alkalinity buffers against a reduction in pH by neutralizing acids. But most people don't realize that alkalinity also carbonates the water and is the reason pH rises naturally.
- Third, pH cannot controlled, but its rise can be limited by Henry's Law of physics. With enough acid and/or CO2 injection, the pH can be suppressed. Using physics for pH containment is a much more economical and pragmatic approach.
- Fourth, pH controls chlorine strength (%HOCl) in pools without cyanuric acid. If your pool has any CYA, that does not apply.
- And finally, the amount of CO2 dissolved in water determines your pH, and it will naturally off-gas to equalize with the air above the pool. This means your pH is supposed to rise up until equilibrium is reached. We can determine that equilibrium point based on the water's carbonate alkalinity level.
If you want to slow the rise in pH and use less chemicals, start by reducing your alkalinity. Make sure to compensate for the lower alkalinity with higher calcium hardness to maintain LSI balance.
1 Lim, L. T., Ah-Kee, E. Y., & Collins, C. E. (2014). Common eye drops and their implications for pH measurements in the management of chemical eye injuries. International journal of ophthalmology, 7(6), 1067–1068. https://doi.org/10.3980/j.issn.2222-3959.2014.06.29
2 This study from 1981 examined 44 people, directly measuring the pH of their tears throughout the day. They found the normal range of pH to be between 6.5 and 7.6, with a mean of 7.0.
3 Each country in Europe has its own standards, but they are generally similar. Germany, France, Italy and the UK allow for lower pH levels than we do in the United States, while Spain allows for both a lower pH and a pH up to 8.0 (which is higher than our standards as they exist today). The Europeans, however, focus on limiting the free chlorine levels. In Germany, for instance, they use the DIN standard, in which free chlorine must be maintained between 0.3-0.6 mg/L (ppm).
Yang, L., Chen, X., She, Q., et.al. (2018). Regulation, formation, exposure, and treatment of disinfection byproducts (DBPs) in swimming pool waters: a critical review. Environment International. Vol. 121(2), pp.1039-1057.
4 Alkalinity tends to rise in pools using CO2 injectors. This occurs because of the excess hydroxides in both liquid chlorine and calcium hypochlorite. When acid is used, these excess hydroxides go unnoticed because they are neutralized easily by acid. But when acid is not used, they accumulate and contribute to total alkalinity. Thus, the alkalinity levels rise. This does not occur in pools using acidic chlorine types like trichlor...but hopefully you're not using trichlor as a primary chlorine on a commercial pool in the first place. Especially not an indoor pool.
5 Wojtowicz, J. (2001). Factors affecting the calcium carbonate saturation index. Journal of the Swimming Pool and Spa Industry. Vol. 1(2), pg. 66.
6 Technically the pH is the concentration of Hydrogen ions. But since we have carbonate alkalinity, the carbonates themselves are bound to Hydrogen and serve as a good proxy for the pH of the water. So instead of looking at the H in carbonic acid, bicarbonate ions and carbonate ions (in the chart above), look the substances more closely. You will see there is a CO2 in each of them, albeit in a different form (CO3). Carbonic acid itself is H2CO3, which is dissolved carbon dioxide (H2O + CO2 ⇌ H2CO3). To lower pH, we must increase the amount of dissolved CO2 in the water (to create more carbonic acid). To increase pH, we must decrease the amount of CO2 in thew water. It will escape naturally due to aeration and CO2 off-gassing.
7 The "strength" of chlorine is usually defined as the percentage of free chlorine in its active killing form, Hypochlorous Acid (HOCl). The expression is %HOCl. In water without any cyanuric acid (CYA), the pH controls this percentage. The lower the pH, the higher the %HOCl relative to its weaker counterpart, Hypochlorite ion (OCl-). In outdoor pools with CYA, however, the pH only impacts the small percentage of chlorine that is not bound to CYA, which is below 3% of the free chlorine in the water. In other words, in stabilized pools, the pH no longer controls the strength of chlorine in a meaningful way. The amount of cyanuric acid does.

 By
By